Adult Gynecology: Reproductive Years
Bs. Nguyễn Hồng Anh
KEY POINTS
1 The causes of abnormal bleeding vary by age, with anovulatory bleeding most likely
in adolescents and perimenopausal women.
2 Anatomic causes of abnormal bleeding including endometrial polyps and leiomyoma
occur more frequently in women of reproductive age than in women in other age
465groups
3 Pelvic masses in adolescents and women of reproductive age are most commonly
functional or benign neoplastic ovarian masses, whereas the risks of malignant
ovarian tumors increase with age.
4 Although pelvic ultrasonography is an excellent technique for imaging pelvic masses
and ultrasonographic characteristics may suggest reassuring characteristics of an
ovarian mass, the possibility of malignancy must be kept in mind.
5 Most uterine leiomyomas are asymptomatic, although bleeding, pressure symptoms,
or pain may necessitate medical or surgical management.
Benign gynecologic conditions can present with signs and symptoms that vary by
age. In this chapter, the most likely causes of specific signs and symptoms,
diagnosis, and management are described for reproductive age and
postmenopausal women. Common gynecologic problems include those that
cause pain and bleeding, such as pelvic masses (which may be symptomatic
or asymptomatic), and vulvar and vaginal symptoms. Benign conditions of the
female genital tract include anatomic lesions of the uterine corpus and cervix,
ovaries, fallopian tubes, vagina, and vulva. A classification of benign lesions of
the vulva, vagina, and cervix appears in Table 10-1. Leiomyoma, polyps, and
hyperplasia are the most common benign conditions of the uterus in adult women.
Benign uterine leiomyoma (uterine fibroids) are presented in Chapter 11. Benign
tumors of the ovaries are listed in Table 10-2. Malignant diseases are presented in
Chapters 37 to 42. Pediatric and adolescent conditions are discussed in Chapter 9.
Table 10-1 Classification of Benign Conditions of the Vulva, Vagina, and Cervix
Vulva
Skin conditions
Pigmented lesions
Tumors and cysts
Ulcers
Nonneoplastic epithelial disorders
Vagina
Embryonic origin
Mesonephric, paramesonephric, and urogenital sinus cysts
466Adenosis (related to in utero diethylstilbestrol exposure)
Vaginal septa or duplications
Pelvic organ prolapse/disorders of pelvic support
Anterior vaginal prolapse
Cystourethrocele
Cystocele
Apical vaginal prolapse
Uterovaginal
Vaginal vault
Posterior vaginal prolapse
Enterocele
Rectocele
Other
Condyloma
Urethral diverticula
Fibroepithelial polyp
Vaginal endometriosis
Cervix
Infectious
Condyloma
Herpes simplex virus ulceration
Chlamydial cervicitis
Other cervicitis
Other
467Endocervical polyps
Nabothian cysts
Columnar epithelium eversion
Table 10-2 Benign Ovarian Tumors
Functional
Follicular
Corpus luteum
Theca lutein
Inflammatory
Tubo-ovarian abscess or complex
Neoplastic
Germ cell
Benign cystic teratoma
Other and mixed
Epithelial
Serous cystadenoma
Mucinous cystadenoma
Fibroma
Cystadenofibroma
Brenner tumor
Mixed tumor
Other
Endometrioma
468REPRODUCTIVE AGE GROUP
Abnormal Bleeding
Normal Menses
After adolescence, menstrual cycles generally conform to a cycle length of 21
to 35 days, with the duration of menstrual flow fewer than 7 days (1). [1] As a
woman approaches menopause, the cycle length becomes more irregular
because fewer cycles are ovulatory (1,2). The most frequent cause of
irregular bleeding in the reproductive age group is hormonal, although other
causes such as pregnancy-related bleeding (spontaneous abortion, ectopic
pregnancy) should always be considered (Table 10-3). A variety of imprecise
terms such as menorrhagia or menometrorrhagia have been used to describe
abnormal uterine bleeding (AUB); it is strongly recommended that these
confusing terms be abandoned in favor of simple designations of menstrual
cycles, describing cycle regularity, frequency, duration, and heaviness of flow
(Table 10-4) (3,4). The International Federation of Gynecology and Obstetrics
(FIGO) and the American College of Obstetricians and Gynecologists (ACOG)
have recommended that systematized nomenclature, the PALM-COEIN acronym,
be used to describe abnormal menses (Table 10-5). The term dysfunctional
uterine bleeding (DUB) should no longer be used (3–6).
Prospective charting of bleeding can be helpful in characterizing abnormal
bleeding. The mean duration of menses is 4.7 days; 89% of cycles last 7 days
or longer. The average blood loss per cycle is 35 mL (6). Menses comprises a
suspension of blood- and tissue-derived solids within a mixture of serum and
cervicovaginal fluid; the blood content of menses varies over the days of
bleeding, but on average is close to 50% (7). Heavy menstrual bleeding is
defined as greater than 80 mL per day, which will result in anemia if recurrent (8).
Pregnancy-Related Bleeding
Pregnancy should always be excluded in women of reproductive age
presenting with AUB.
Table 10-3 Causes of Bleeding by Approximate Frequency and Age Group
469Spontaneous abortion can be associated with excessive or prolonged bleeding.
A woman may be unaware that she conceived and may seek care because of
abnormal bleeding. In the United States, nearly half of pregnancies are
unintended. These women may be at particular risk for bleeding related to an
unsuspected pregnancy. About one-half of unintended pregnancies result from
nonuse of contraception; the other one-half result from contraceptive failures (9).
[1] Unintended pregnancies are most likely to occur among adolescents and
women older than 40 years of age (see Chapter 14). If an ectopic pregnancy is
ruled out, the management of spontaneous abortion may include either
observation, if the bleeding is not excessive; medical or pharmacologic uterine
evacuation (with misoprostol); or surgical management with suction curettage or
dilation and curettage (D&C), depending on the clinician’s judgment and the
patient’s preference (10).
Differential Diagnosis of Abnormal Bleeding
Structural causes of AUB include the PALM in PALM-COEIN (Polyps,
Adenomyosis, Leiomyoma, Malignancy/Hyperplasia). [2] Anatomic causes of
abnormal bleeding occur more frequently in women of reproductive age than
in women in other age groups. [5] Uterine leiomyomas and endometrial
polyps are common conditions that most often are asymptomatic; however,
they remain important causes of abnormal bleeding (11).
Polyps, AUB-P
[2] Endometrial polyps are a cause of intermenstrual bleeding, heavy
menstrual bleeding, irregular bleeding, and postmenopausal bleeding. They
are associated with the use of tamoxifen and infertility, and can cause
dysmenorrhea. As with leiomyomas, most endometrial polyps are
asymptomatic. [2] The incidence of endometrial polyps increases with age
throughout the reproductive years (12). The diagnosis may be suspected on the
basis of endometrial thickening on transvaginal pelvic ultrasound, and vascular
470patterns of feeder blood vessels may aid in distinguishing endometrial polyps
from intracavity fibroids and from endometrial malignancy (13–15). Confirmation
of a polyp requires visualization with hysteroscopy, sonohysterography, or the
microscopic assessment of tissue obtained by a biopsy done in the office or with a
D&C. Whether and when to recommend removal is not well established,
particularly if a polyp is asymptomatic and is found incidentally. The effect of
polyps on fertility is not clear, though there is evidence that removal may improve
rates of pregnancy in infertile patients (16). One study of randomly selected
Danish women using transvaginal ultrasound and sonohysterography found
polyps in 5.8% of asymptomatic premenopausal women and 11.8% of
asymptomatic postmenopausal women. In this study, abnormal bleeding was
present in 38% of those without polyps versus 13% with polyps (15). Endometrial
polyps can regress spontaneously, although it is not clear how frequently this
occurs. In one study of asymptomatic women, the 1-year regression rate was 27%
(17). Smaller polyps are more likely to resolve, and larger polyps may be more
likely to result in abnormal bleeding (18). Whereas polyps may resolve
spontaneously over time, a clinically important question is whether they are likely
to undergo malignant transformation. Because even asymptomatic polyps are
usually removed at the time of identification, this question is difficult to answer.
[2] The chance of malignancy or premalignant changes in endometrial polyps
appears to be quite low in premenopausal women and higher among
postmenopausal women, with bleeding reports that range from 0.2% to 24% in
premalignant change and 0% to 13% in malignancy (16).
Table 10-4 Menstrual Terminology
Table 10-5 Abnormal Uterine Bleeding Terminology
Structural Causes PALM
AUB-P Polyp
AUB-A Adenomyosis
AUB-L Leiomyoma
471AUB-M Malignancy + Hyperplasia
Nonstructural COEIN
AUB-C Coagulopathy
AUB-O Ovulatory dysfunction
AUB-E Endometrial
AUB-I Iatrogenic
AUB-N Not yet classified
Adenomyosis, AUB-A
Traditionally adenomyosis has been diagnosed by histology at the time of
hysterectomy, making estimates of prevalence and contribution to AUB and
pelvic pain unclear. With improving imaging technology and evolving
diagnostic criteria for adenomyosis on ultrasound and MRI, adenomyosis
can be diagnosed prior to hysterectomy and is included as a structural cause
of abnormal bleeding. The incidence of incidentally identified adenomyosis on
pelvic imaging is not yet known (5).
Leiomyoma, AUB-L
[5] Uterine leiomyomas occur in as many as one-half of all women older than age
35 years and are the most common tumors of the genital tract (12). The incidence
varies from 30% to 70%, depending on the criteria for study, whether clinical
symptoms, ultrasound, or histologic assessment (11). One study of a randomly
selected population estimated a cumulative prevalence of greater than 80% in
black women and nearly 70% in white women based on ultrasound (19).
Abnormal bleeding is the most common symptom for women with leiomyomas
who are symptomatic. Although the number and size of uterine leiomyomas
do not appear to influence the occurrence of abnormal bleeding, submucosal
myomas are the most likely to cause bleeding. The mechanism of abnormal
bleeding related to leiomyomas is not well established (see Chapter 11 for further
discussion of uterine fibroids).
Malignancy and Hyperplasia, AUB-M
Unopposed estrogen is associated with a variety of abnormalities of the
endometrium, from cystic hyperplasia to adenomatous hyperplasia, hyperplasia
with cytologic atypia, and invasive carcinoma. Abnormal bleeding is the most
frequent symptom of women with invasive cervical cancer. A visible cervical
472lesion should be evaluated by biopsy rather than awaiting the results of cervical
cytology testing, because those results may be falsely negative with invasive
lesions as caused by tumor necrosis. Although vaginal neoplasia is uncommon,
the vagina should be evaluated carefully when abnormal bleeding is present.
Attention should be directed to all surfaces of the vagina, including anterior and
posterior areas that may be obscured by the vaginal speculum on examination.
Nonstructural causes of AUB include the COEIN in PALM-COEIN
(Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, NOS)
Coagulopathy, AUB-C
As with adolescents, hematologic causes of abnormal bleeding should be
considered in women with heavy menstrual bleeding, particularly in those who
have had heavy bleeding since menarche. Of all women with menorrhagia, 5%
to 20% have a previously undiagnosed bleeding disorder, primarily the von
Willebrand disease (20). Table 10-6 presents guidelines for a gynecologist’s
suspicion of a bleeding disorder and pursuit of a diagnosis (21). Abnormal liver
function, which can be seen with alcoholism or other chronic liver diseases,
results in inadequate production of clotting factors and can lead to excessive
menstrual bleeding.
Ovulatory Dysfunction, AUB-O
[1] Most anovulatory bleeding results from what is termed estrogen
breakthrough. In the absence of ovulation and the production of progesterone, the
endometrium responds to estrogen stimulation with proliferation. This
endometrial growth without periodic shedding results in eventual breakdown of
the fragile endometrial tissue. Healing within the endometrium is irregular and
dyssynchronous. Relatively low levels of estrogen stimulation will result in
irregular and prolonged bleeding, whereas higher sustained levels result in
episodes of amenorrhea followed by acute, heavy bleeding.
Many ovulatory disorders relate to endocrine disturbances. Both
hypothyroidism and hyperthyroidism can be associated with abnormal
bleeding. With hypothyroidism, menstrual abnormalities, including
menorrhagia, are common (see Chapter 35). The most common cause of
thyroid hyperfunctioning in premenopausal women is Graves’ disease, which
occurs four to five times more often in women than men. Hyperthyroidism
can result in oligomenorrhea or amenorrhea, and it can lead to elevated
levels of plasma estrogen (22). Other causes of anovulation include
hypothalamic dysfunction, hyperprolactinemia, premature ovarian failure
(POF), and primary pituitary disease (Table 10-7). These conditions often
are considered causes of amenorrhea, but they may be the cause of irregular
473bleeding (see Chapter 34). The rare and unusual causes of abnormal bleeding
should not be overlooked. Women with primary ovarian insufficiency (POI;
previously termed POF) frequently see several clinicians with symptoms of
oligomenorrhea or amenorrhea prior to receiving a diagnosis; the diagnosis
of POI is often delayed during waning ovarian function and insufficiency
(23,24). POI is thought to occur in approximately 1 of 100 women by age 40, 1 of
1,000 women by age 30, and 1 of 10,000 women by age 20. Women should be
encouraged to track their menstrual cyclicity and to consider that the menstrual
cycle can be a “vital sign” that reflects the overall health (25).
Table 10-6 When Should a Gynecologist Suspect a Bleeding Disorder
Heavy menstrual bleeding since menarche
Family history of bleeding disorder
Personal history of any of the following:
Epistaxis in the last year
Bruising without injury >2-cm diameter
Minor wound bleeding
Oral or gastrointestinal bleeding without anatomic lesion
Prolonged or heavy bleeding after dental extraction
Unexpected postoperative bleeding
Hemorrhage from ovarian cyst
Hemorrhage requiring blood transfusion
Postpartum hemorrhage, especially delayed >24 h
Failure to respond to conventional management of menorrhagia
From James AH, Kouides PA, Abdul-Kadir R, et al. von Willebrand disease and other
bleeding disorders in women: Consensus on diagnosis and management from an
international expert panel. Am J Obstet Gynecol 2009;201:12e1–e8.
Table 10-7 Conditions Associated with Anovulation and Abnormal Bleeding
Eating disorders
474Anorexia nervosa
Bulimia nervosa
Excessive physical exercise
Chronic illness
Primary ovarian insufficiency—POI (previously termed premature ovarian failure
[POF])
Alcohol and other drug abuse
Stress
Thyroid disease
Hypothyroidism
Hyperthyroidism
Diabetes mellitus
Androgen excess syndromes (e.g., polycystic ovary syndrome [PCOS])
Diabetes mellitus can be associated with anovulation, obesity, insulin
resistance, and androgen excess. Androgen disorders are very common among
women of reproductive age and should be evaluated and managed accordingly.
Polycystic ovary syndrome (PCOS) is present in 5% to 8% of adult women and
undiagnosed in many of them (26). Because androgen disorders are associated
with significant cardiovascular disease, the condition should be diagnosed
promptly and treated. This condition becomes of more immediate concern in
women of reproductive age because of concerns related to fertility. Management
of bleeding disorders associated with androgen excess consists of an appropriate
diagnostic evaluation followed by the use of oral contraceptives (in the absence of
significant contraindications or the desire for conception) or the use of insulinsensitizing agents, coupled with dietary and exercise modification (27,28).
Endometrial, AUB-E
In ovulatory cycles, the endometrium itself may contribute to abnormal or
heavy menstrual bleeding (AUB/HMB). There is evidence that deficiencies of
vasoconstrictors or excess of vasodilators may lead to heavy bleeding. Local
vasoconstrictors include endothelin-1 and prostaglandin F2alpha. Local
vasodilators include prostacyclin I2 and prostaglandin E2 (29). Inflammation and
475infection can affect the endometrium. Menorrhagia can be the first sign of
endometritis in women infected with sexually transmissible organisms.
Women with cervicitis, particularly chlamydial cervicitis, can experience
irregular bleeding and postcoital spotting (see Chapter 15). Cervical testing for
Chlamydia trachomatis should be considered, especially for adolescents, women
in their 20s, and women who are not in a monogamous relationship. Endometritis
can cause excessive menstrual flow. A woman who seeks treatment for
menorrhagia and increased menstrual pain and has a history of light-to-moderate
previous menstrual flow may have an upper genital tract infection or pelvic
inflammatory disease (PID) (endometritis, salpingitis, oophoritis). Occasionally,
chronic endometritis will be diagnosed when an endometrial biopsy is obtained
for evaluation of abnormal bleeding in a patient without specific risk factors for
PID.
Iatrogenic, AUB-I
Iatrogenic-Exogenous Hormones
Irregular bleeding that occurs while a woman is using contraceptive
hormones should be considered in a different context than bleeding that
occurs in the absence of exogenous hormone use. Breakthrough bleeding
during the first 1 to 3 months of oral contraceptive use occurs in as many as
30% to 40% of users; it should almost always be managed expectantly with
reassurance because the frequency of breakthrough bleeding decreases with
each subsequent month of use (30). Irregular bleeding can result from
inconsistent use (31,32). Other estrogen–progestin delivery systems, including the
contraceptive patch, vaginal ring, and intramuscular regimens, are associated with
irregular breakthrough bleeding. These nondaily contraceptive regimens may
promote successful use, making irregular bleeding a less important factor for
some women in assessing the balance of risks versus benefits (see Chapter 14).
The use of progestin-only methods—including depot medroxyprogesterone
acetate (DMPA), progestin-only pills, the contraceptive implant, and the
levonorgestrel intrauterine system (IUS)—is associated with relatively high rates
of initial irregular and unpredictable bleeding; rates of amenorrhea vary over time
and by method (33). Counseling about the frequent side effects of irregular
bleeding is imperative before initially prescribing these methods of contraception.
Women who do not believe that they can cope with irregular, unpredictable
bleeding may not be good candidates for these methods. Hormonal implants and
IUDs releasing progestins do offer significant benefits of high efficacy and ease
of use (34,35). The management of irregular bleeding with hormonal
contraceptive use can range from reassurance and initial expectant
476management to recommendations for a change in the hormonal delivery
system or regimen. The use of additional oral estrogen or combined oral
contraceptives for 10 to 20 days improves bleeding with both DMPA and the
subdermal levonorgestrel in some studies (36). The use of a 5- to 7-day course of
NSAIDs may result in decreased breakthrough bleeding (36). The development of
a better understanding of the mechanisms causing irregular bleeding will likely
result in more effective and acceptable management strategies (33).
Not all bleeding that occurs while an individual is using hormonal
contraception is a consequence of hormonal factors. In one study, women who
experienced irregular bleeding while taking oral contraceptives had a higher
frequency of C. trachomatis infection (37). Screening for sexually transmitted
infections (STIs) should be considered in women presenting with irregular
bleeding while using hormonal contraception.
Not Yet Classified, AUB-N
This includes causes of AUB not yet discovered and those rarer and lessunderstood causes, including myometrial hypertrophy and AV malformations (8).
Diagnosis of Abnormal Bleeding
For all women, the evaluation of excessive and abnormal menses includes a
thorough medical and gynecologic history, the exclusion of pregnancy, the
consideration of possible malignancy, and a careful gynecologic examination.
Abnormal bleeding, either intermenstrual or postcoital, can be caused by
cervical lesions. Bleeding can result from endocervical polyps and infectious
cervical lesions, such as condylomata, herpes simplex virus ulcerations,
chlamydial cervicitis, or cervicitis caused by other organisms. Other benign
cervical lesions, such as wide eversion of endocervical columnar epithelium or
nabothian cysts, may be detected on examination, but rarely cause bleeding.
For women of normal weight between the ages of approximately 20 and 35
years who do not have clear risk factors for STDs, have no signs of androgen
excess, are not using exogenous hormones, and have no other findings on
examination, management may be based on a clinical diagnosis. Additional
laboratory or imaging studies may be indicated if the diagnosis is not apparent on
the basis of examination and history.
Laboratory Studies
In any patient with heavy menstrual bleeding, an objective measurement of
hematologic status should be performed with a complete blood count to
detect anemia or thrombocytopenia. A pregnancy test should be performed
477to rule out pregnancy-related problems. A TSH level and chlamydia testing
should be considered. Because of the possibility of a primary coagulation
problem, screening coagulation studies should be ordered where appropriate
(Table 10-6). The consensus report of an international expert panel recommends
measurement of CBC, platelet count and function, PT, activated PTT, VWF
(measured with ristocetin cofactor activity and antigen, factor VIII), and
fibrinogen to be assessed in collaboration with a hematologist (38).
Imaging Studies
Women with abnormal bleeding who have a history consistent with chronic
anovulation, are obese, or older than 35 to 40 years of age, require further
evaluation. A pelvic ultrasonographic examination may be helpful in delineating
anatomic abnormalities if the examination results are suboptimal or if an ovarian
mass is suspected. A pelvic ultrasonographic examination is the best initial
technique for evaluating the uterine contour, endometrial thickness, and ovarian
structure (39,40). The use of a vaginal probe transducer allows assessment of
endometrial and ovarian disorders, particularly in women who are obese. Because
of variation in endometrial thickness with the menstrual cycle,
measurements of endometrial stripe thickness are significantly less useful in
premenopausal than postmenopausal women (41). Sonohysterography is
especially helpful in visualizing intrauterine problems such as polyps or
submucosal leiomyoma. Although these sonographic techniques are helpful in
visualizing intrauterine pathology, histologic evaluation is required to rule out
malignancy. Other techniques, such as CT scanning and MRI, are not as helpful
in the initial evaluation of causes of abnormal bleeding and should be reserved for
specific indications, such as exploring the possibility of other intra-abdominal
pathology or adenopathy. MRI can be a secondary step in evaluating the location
of uterine fibroids with relationship to the endometrial cavity, staging and
preoperative evaluation of endometrial cancer, detecting adenomyosis, and
delineating adnexal and ovarian pathology (42).
Endometrial Sampling
[1] Endometrial sampling should be performed to evaluate abnormal
bleeding in women who are at risk for endometrial pathology, including
polyps, hyperplasia, or carcinoma. Such sampling is mandatory in the
evaluation of anovulatory bleeding in women older than 45 or in younger
women who are obese, in those who do not respond to medical therapy or
those with a history of prolonged anovulation (10).
The technique of a D&C, which was previously used extensively for the
evaluation of abnormal bleeding, has now been largely replaced by
478endometrial biopsy in the office. The classic study in which a D&C was
performed before hysterectomy with the conclusion that less than one-half of the
endometrium was sampled in more than one-half of the patients led to questioning
the use of D&C for endometrial diagnosis (43,44). Hysteroscopy, either
diagnostic or operative, with endometrial sampling, can be performed either in the
office or operating room (45).
A number of devices are designed for endometrial sampling, including a
commonly used, inexpensive, disposable, flexible plastic sheath with an internal
plunger that allows tissue aspiration; disposable plastic cannulae of varying
diameters that attach to a manually locking syringe that allows the establishment
of a vacuum; and cannulae (both rigid metal and plastic) with tissue traps that
attach to an electric vacuum pump (Fig. 10-1). Several studies comparing the
adequacy of sampling using these devices with D&C showed a comparable ability
to detect abnormalities. It should be noted that these devices are designed to
obtain a tissue sample rather than a cytologic washing. The diagnostic accuracy of
endometrial biopsy for endometrial malignancy and hyperplasia is good, although
persistent bleeding should prompt further testing (46). Hysteroscopy with targeted
biopsies is more sensitive than a D&C in evaluating uterine pathology (29).
FIGURE 10-1 Devices used for sampling endometrium. Top: Kevorkian curette. Bottom:
Pipelle.
Management of Abnormal Bleeding
Attention should be directed to establishing a cause of abnormal bleeding. In
most cases, medical therapy is effective in managing abnormal bleeding and
should be attempted before surgical management. [1] Medical management
479with either combined hormonal contraceptives or progestins is the preferred
therapy of anovulatory bleeding in women of reproductive age (8). Progestinreleasing IUDs are effective in treating heavy menstrual bleeding and demonstrate
comparable benefits for the quality of life (47). It is argued that the IUD should
be offered prior to consideration of hysterectomy, as there are comparable
benefits on heavy menstrual bleeding and clear cost benefits (48). When
medical therapy fails in women with anovulatory uterine bleeding and without the
desire for future childbearing, the surgical options of endometrial ablation or
hysterectomy can be considered. Endometrial ablation is an efficient and costeffective alternative to hysterectomy, although this therapy may not be definitive,
with increasing rates of repeat ablation and hysterectomy over time (8). In women
with leiomyomas, hysterectomy provides a definitive cure. A variety of surgical
alternatives to hysterectomy are available to women with symptomatic uterine
leiomyomas (see Chapter 11).
Nonsurgical Management
Most bleeding problems, including anovulatory bleeding, can be managed
nonsurgically. Treatment with NSAIDs, such as ibuprofen and mefenamic acid,
decreases menstrual flow by 30% to 50%, but is less effective than tranexamic
acid, danazol, or levonorgestrel IUD (49). Antifibrinolytics, such as tranexamic
acid, are effective in reducing menstrual blood loss, and this indication was
approved by the FDA in late 2008 (50).
Hormonal management of abnormal bleeding can frequently control
excessive or irregular bleeding. The treatment of choice for anovulatory
bleeding is medical therapy with combined oral contraceptives or progestins
including the levonorgestrel IUD (5). Oral contraceptives are used clinically to
decrease menstrual flow, although supporting data from prospective clinical trials
are sparse (51). Low-dose oral contraceptives may be used by reproductive age
women without medical contraindications and during the perimenopausal years in
healthy nonsmoking women who have no major cardiovascular risk factors. The
benefits of menstrual regulation in such women often override the potential risks.
The medical treatment of acute abnormal bleeding in reproductive age women is
the same as that described for adolescents (see Chapter 9).
For patients in whom estrogen use is contraindicated, progestins, both oral
and parenteral, can be used to control excessive bleeding. Cyclic oral
medroxyprogesterone acetate, administered from day 15 or 19 to day 26 of the
cycle, reduces menstrual flow but offers no advantages over other medical
therapies, such as NSAIDs, tranexamic acid, danazol, or the levonorgestrel IUD;
progestin therapy for 21 days of the cycle reduces menstrual flow, although
women found the treatment less acceptable than the levonorgestrel IUD (52). The
480benefits of progestins to the patient with oligomenorrhea and anovulation include
a regular flow and the prevention of long intervals of amenorrhea, which may end
in unpredictable, profuse bleeding. This therapy reduces the risk of hyperplasia
resulting from persistent, unopposed estrogen stimulation of the endometrium.
Depot formulations of medroxyprogesterone acetate, oral progestins,
levonorgestrel IUDs, and combined oral contraceptives are used to establish
amenorrhea in women at risk of excessive bleeding (53). Oral, parenteral, or
intrauterine delivery of progestins is used in selected women with endometrial
hyperplasia or early endometrial cancer who wish to maintain their fertility or in
whom surgical risks are judged to be prohibitive (29). Continued monitoring with
repeated sampling is indicated. Danazol is effective in decreasing bleeding and
inducing amenorrhea; it is rarely used for ongoing management of abnormal
bleeding because of its androgenic side effects, including weight gain, hirsutism,
alopecia, and irreversible voice changes. GnRH analogs are used for short-term
treatment of abnormal bleeding, either alone or with add-back therapy consisting
of combined estrogen/progestogen or progestogen alone (54).
Surgical Therapy
The surgical management of abnormal bleeding should be reserved for
situations in which medical therapy is unsuccessful or is contraindicated.
Although sometimes appropriate as a diagnostic technique, D&C is
questionable as a therapeutic modality. One study reported a measured
reduction in menstrual blood loss for the first menstrual period only (55). Other
studies suggest a longer-lasting benefit (56).
The surgical options range from a variety of techniques for endometrial
ablation or resection, to hysterectomy or a variety of conservative surgical
techniques for the management of uterine leiomyoma, including
hysteroscopy with resection of submucous leiomyomas, laparoscopic and
robotic techniques of myomectomy, uterine artery embolization, and
magnetic resonance–guided focused ultrasonography ablation (see Chapters
26 and 27). The choice of procedure depends on the cause of bleeding, the
patient’s preferences, the physician’s experience and skills, the availability of
newer technologies, and a careful assessment of risks versus benefits based on the
patient’s medical condition, concomitant gynecologic symptoms or conditions,
and desire for future fertility. The assessment of the relative advantages, risks,
benefits, complications, and indications of these procedures is a subject of
ongoing clinical research. Various techniques of endometrial ablation were
compared with the gold standard of endometrial resection, and the evidence
suggests comparable success rates and complication profiles (57). The advantages
of techniques other than hysterectomy include a shorter recovery time and
481reduced early morbidity. Symptoms can recur or persist and repeat procedures or
subsequent hysterectomy may be required if conservative options are chosen.
Additional studies that include quality-of-life outcomes will be helpful.
Collaborative decision making, taking into account individual patient preferences,
should follow a thorough discussion of options, risks, and benefits (58,59). Much
is written about the psychological sequelae of hysterectomy, and some of the
aforementioned surgical techniques were developed in an effort to provide less
drastic management options. Most well-controlled studies suggest that, in the
absence of pre-existing psychopathology, indicated but elective surgical
procedures for hysterectomy have few, if any, significant psychological
sequelae (including depression) (see Chapters 23 and 27) (60,61).
Pelvic Masses
[3] Conditions diagnosed as a pelvic mass in women of reproductive age are
presented in Table 10-8.
Differential Diagnosis
It is difficult to determine the frequency of diagnoses of pelvic mass in women of
reproductive age because many pelvic masses are not treated with surgery.
Nonovarian or nongynecologic conditions may be confused with an ovarian
or uterine mass (Table 10-8). The frequency of masses found at laparotomy
has been studied, although the percentages are affected by varying
indications for surgery, indications for referral, type of practice (gynecologic
oncology vs. general gynecology), and patient populations (e.g., a higher
percentage of African Americans with uterine leiomyomas). Benign masses,
such as functional ovarian cysts or asymptomatic uterine leiomyoma,
typically do not require or warrant surgery (Table 10-9).
Table 10-8 Conditions Diagnosed as a Pelvic Mass in Women of Reproductive Age
Urinary
Full urinary bladder
Urachal cyst
Uterus
Sharply anteflexed or retroflexed uterus
Intraligamentous leiomyomas
482Pregnancy (with or without concomitant leiomyomas)
Intrauterine
Tubal
Abdominal
Ovarian or adnexal masses
Functional cysts
Neoplastic tumors
Benign
Malignant
Inflammatory masses
Tubo-ovarian complex
Diverticular abscess
Appendiceal abscess
Other
Matted bowel and omentum
Peritoneal cyst
Stool in sigmoid
Paraovarian or paratubal cysts
Less common conditions that must be excluded:
Pelvic kidney
Carcinoma of the colon, rectum, appendix
Carcinoma of the fallopian tube
Retroperitoneal tumors (anterior sacral meningocele)
Uterine sarcoma or other malignant tumors
Table 10-9 Causes of Pelvic Mass by Approximate Frequency and Age
483Age is an important determinant of the likelihood of malignancy. In one
study of women who underwent laparotomy for pelvic mass, malignancy was
seen in only 10% of those younger than 30 years of age, and most of these
tumors had low malignancy potential (62). The most common tumors found
during laparotomy for pelvic mass are mature cystic teratomas or dermoids
(seen in one-third of women younger than 30 years of age) and
endometriomas (approximately one-fourth of women 31 to 49 years of age)
(62).
Uterine Masses
Uterine leiomyomas, commonly termed uterine fibroids, are by far the most
common benign uterine tumors and are usually asymptomatic. Other benign
uterine growths, such as uterine vascular tumors, are rare. See Chapter 11 for
discussion of diagnosis, types and locations of fibroids, incidence, symptoms,
causes, natural history, pathology, and management.
Ovarian Masses
[3] During the reproductive years, the most common ovarian masses are benign.
Ovarian masses can be functional or neoplastic, and neoplastic tumors can be
benign or malignant. Functional ovarian masses include follicular and corpus
luteal cysts. About two-thirds of ovarian tumors are encountered during the
reproductive years. Most ovarian tumors (80% to 85%) are benign, and two-thirds
of these occur in women between 20 and 44 years of age. The chance that a
primary ovarian tumor is malignant in a patient younger than 45 years of
age is less than 1 in 15. Most tumors produce few or only mild, nonspecific
symptoms. The most common symptoms include abdominal distention,
abdominal pain or discomfort, lower abdominal pressure sensation, and urinary or
gastrointestinal symptoms. If the tumor is hormonally active, symptoms of
hormonal imbalance, such as vaginal bleeding related to estrogen production, may
be present. Acute pain may occur with adnexal torsion, cyst rupture, or bleeding
into a cyst. Pelvic findings in patients with benign and malignant tumors may
484differ. Masses that are unilateral, cystic, mobile, and smooth are most likely to be
benign, whereas those that are bilateral, solid, fixed, irregular, and associated with
ascites, cul-de-sac nodules, and a rapid rate of growth are more likely to be
malignant (63).
In assessing ovarian masses, the distribution of primary ovarian neoplasms by a
decade of life can be helpful. Ovarian masses in women of reproductive age are
most likely benign, but the possibility of malignancy must be considered (Fig. 10-
2).
FIGURE 10-2 Preoperative evaluation of the patient with an adnexal mass.
Nonneoplastic Ovarian Masses
[3] Functional ovarian cysts include follicular cysts, corpus luteum cysts, and
theca lutein cysts. All are benign and usually do not cause symptoms or
require surgical management. Cigarette and marijuana smoking are associated
486with an increased risk of functional cysts, although the increased risk may be
attenuated in overweight or obese women (64). Oral contraceptive use is
associated with a decreased risk of developing functional ovarian cysts,
although low-dose pills may have a smaller benefit. Oral contraceptives do
not hasten the resolution of ovarian cysts (65,66). The annual rate of
hospitalization for functional ovarian cysts is estimated to be as high as 500 per
100,000 woman-years in the United States, although little is known about the
epidemiology of the condition. [3] The most common functional cyst is the
follicular cyst, which is rarely larger than 8 cm. A cystic follicle can be defined
as a follicular cyst when its diameter is greater than 3 cm. These cysts usually are
found incidental to pelvic examination or pelvic imaging, although they may
rupture or torse, causing pain and peritoneal signs. They typically resolve in 4 to 8
weeks with expectant management (66).
Corpus luteum cysts are less common than follicular cysts. Corpus luteum
cysts may rupture, leading to a hemoperitoneum and requiring surgical
management. Patients taking anticoagulant therapy or with bleeding diatheses are
at particular risk for hemorrhage and rupture. Rupture of these cysts occurs more
often on the right side and may occur during intercourse. Most ruptures occur
on cycle days 20 to 26 (67). Unruptured corpus luteum cysts can cause pain,
presumably because of bleeding into the enclosed ovarian cyst cavity. They can
produce symptoms and be difficult to discern from adnexal torsion.
Theca lutein cysts are the least common of functional ovarian cysts. They
are usually bilateral and occur with pregnancy, including molar pregnancies.
They may be associated with multiple gestations, molar pregnancies,
choriocarcinoma, diabetes, Rh sensitization, clomiphene citrate use, human
menopausal gonadotropin–human chorionic gonadotropin ovulation induction,
and the use of GnRH analogs. Up to one-quarter of complete molar pregnancies
will have theca lutein cysts which will regress spontaneously (68).
Combination monophasic oral contraceptive therapy is reported to reduce
the risk of developing functional ovarian cysts by suppression of both
follicular development and ovulation (69). It appears that, in comparison with
previously available higher-dose pills, the effect of cyst suppression with lowdose oral contraceptives is attenuated. The use of triphasic oral contraceptives is
not associated with an appreciable increased risk of functional ovarian cysts.
Other Benign Masses
Women with endometriosis may develop ovarian endometriomas (“chocolate”
cysts), which can enlarge from 6 to 8 cm in size. A mass that does not resolve
with observation may be an endometrioma (see Chapter 13). Excision of
endometriosis is preferable to ablative techniques with regard to achievement of
487spontaneous pregnancy (70). New data suggests that women with or without
endometriomas have similar success with conceiving when using ART and do not
need removal prior to fertility treatment if asymptomatic and the diagnosis is not
in question (71,72).
Although enlarged, polycystic ovaries were originally considered the sine qua
non of PCOS, and are included among the Rotterdam diagnostic criteria; they are
not always present with other features of the syndrome (73,74). An enlarged
ovarian volume is suggested as an alternative diagnostic criterion, although what
the threshold should be has been debated (75). The 2003 Rotterdam consensus
uses a volume greater than 10 mL in either ovary or 12 or more subcentimeter
antral follicles in either ovary (74).
The prevalence of PCOS among the general population depends on the
diagnostic criteria used. In one study, 257 volunteers were examined with
ultrasonography; 22% were found to have polycystic ovaries (76). The finding of
generously sized ovaries on examination or polycystic ovaries on
ultrasonographic examination should prompt evaluation for the full-blown
syndrome, which includes hyperandrogenism, chronic anovulation, and polycystic
ovaries (26). Therapy for PCOS is generally medical rather than surgical, with
lifestyle modification and weight loss playing a potentially important role (77).
Neoplastic Masses
[3] Most benign cystic teratomas (dermoid cysts) occur during the
reproductive years in adolescents and young women, although dermoid cysts
have a wider age distribution than other ovarian germ cell tumors; in some
case series, up to 25% of dermoids occur in postmenopausal women, and
they can occur in newborns (78). [3] Histologically, benign cystic teratomas
have an admixture of elements (Fig. 10-3). Malignant transformation occurs in
less than 2% of dermoid cysts in women of all ages; most cases occur in women
older than 40 years of age. The risk of torsion with dermoid cysts is
approximately 15%, and occurs more frequently than with other ovarian tumors,
perhaps because of the high fat content of most dermoid cysts, allowing them to
float within the abdominal and pelvic cavity. As a result of this fat content, on
pelvic examination a dermoid cyst frequently is described as anterior in location.
They are bilateral in approximately 10% of cases, although many have advanced
the argument against bivalving a normal-appearing contralateral ovary because of
the risk of adhesions, which may result in infertility. An ovarian cystectomy is
almost always possible, even if it appears that only a small amount of ovarian
tissue remains. Preserving a small amount of ovarian cortex in a young patient
with a benign lesion is preferable to the loss of the entire ovary (79).
Laparoscopic cystectomy often is possible, and intraoperative spill of tumor
488contents is rarely a cause of complications, although granulomatous peritonitis
has been reported (80). A minimally invasive, fertility-sparing approach is
preferred for benign masses (81).
[3] The risk of epithelial tumors increases with age. Although serous
cystadenomas are often considered the more common benign neoplasm, in one
study, benign cystic teratomas represented 66% of benign tumors in women
younger than 50 years of age; serous tumors accounted for only 20% (82). Serous
tumors are generally benign; 5% to 10% have borderline malignant
potential, and 20% to 25% are malignant. The major risk factor for ovarian
cancer is a family history of ovarian cancer or a familial syndrome,
particularly BRCA1 mutation (approx. 40% risk), BRCA2 mutation (15%
risk) or the Lynch syndrome (5% risk) (81).
Serous cystadenomas are often multilocular, sometimes with papillary
components (Fig. 10-4). The surface epithelial cells secrete serous fluid, resulting
in a watery cyst content. Psammoma bodies, which are areas of fine calcific
granulation, may be scattered within the tumor and are visible on the radiograph.
A frozen section is necessary to distinguish between benign, borderline, and
malignant serous tumors because this distinction cannot be made on gross
examination alone. Mucinous ovarian tumors may grow to large dimensions.
Benign mucinous tumors typically have a lobulated, smooth surface, are
multilocular, and may be bilateral in up to 10% of cases. Mucoid material is
present within the cystic loculations. Five to 10% of mucinous ovarian tumors
are malignant (see Chapter 39). They may be difficult to distinguish
histologically from metastatic gastrointestinal malignancies. Other benign ovarian
tumors include fibromas (a focus of stromal cells), Brenner tumors (which appear
grossly similar to fibromas and are frequently found incidentally), and mixed
forms of tumors, such as the cystadenofibroma.
FIGURE 10-3 Mature cystic teratoma (dermoid cyst) of the ovary.
FIGURE 10-4 Serous cystadenoma.
Uterine, gastric, breast, and colorectal malignancies can metastasize to the
ovaries and should be considered, although as with many malignancies, these
tumors are more common in postmenopausal-aged women.
Other Adnexal Masses
[3] Masses that include the fallopian tube are related primarily to
inflammatory causes in the reproductive age group. A tubo-ovarian abscess
can be present in association with PID (see Chapter 15). A complex inflammatory
mass consisting of the bowel, tube, and ovary may be present without a large
abscess cavity. Ectopic pregnancies can occur in the reproductive age group and
must be excluded when a patient presents with pain, a positive pregnancy test,
and an adnexal mass (see Chapter 32). Paraovarian cysts may be noted on
examination or in imaging studies. In many instances, a normal ipsilateral ovary
can be visualized using ultrasonography. The frequency of malignancy in
paraovarian tumors is quite low and may be more common in paraovarian masses
larger than 5 cm (83).
491Diagnosis of Pelvic Masses
A complete pelvic examination, including rectovaginal examination and
Papanicolaou (Pap) test, should be performed. Estimations of the size of a mass
should be presented in centimeters rather than in comparison to common
objects or fruit (e.g., orange, grapefruit, tennis ball, golf ball). After pregnancy is
excluded, one simple office technique that can help determine whether a mass is
uterine or adnexal includes sounding and measuring the depth of the uterine
cavity. Pelvic imaging can confirm the characteristics of the adnexal mass—
whether solid or cystic or mixed echogenicity. Diagnosis of uterine leiomyomas
usually is based on the characteristic finding of an irregularly enlarged uterus. The
size and location of the usually multiple leiomyomas can be confirmed and
documented with pelvic ultrasonography (Fig. 10-5). If the examination is
adequate to confirm uterine leiomyoma and symptoms are absent,
ultrasonography is not always necessary unless an ovarian mass cannot be
excluded. A fixed or nodular pelvic mass should always raise concern for
malignancy.
FIGURE 10-5 Transvaginal pelvic ultrasound demonstrating multiple uterine leiomyomas.
Other Studies
Endometrial sampling with an endometrial biopsy or hysteroscopy is
492mandatory when both pelvic mass and abnormal bleeding are present. An
endometrial lesion—carcinoma or hyperplasia—may coexist with a benign mass
such as a leiomyoma. In a woman with leiomyomas, abnormal bleeding cannot be
assumed to be caused solely by the fibroids. Clinicians differ in recommendations
about the need for endometrial biopsy when the diagnosis is leiomyomas with
regular menses.
If urinary symptoms are prominent, studies of the urinary tract may be
necessary, including urodynamic testing, if incontinence or symptoms of pelvic
pressure are present. Cystoscopy may be necessary or appropriate to rule out
intrinsic bladder lesions.
Laboratory Studies
Laboratory studies that are indicated for women of reproductive age with a pelvic
mass include pregnancy test, cervical cytology, and complete blood count. The
value of tumor markers, such as CA125 in distinguishing malignant from benign
adnexal masses in premenopausal women with a pelvic mass, is questioned. A
number of benign conditions, including uterine leiomyomas, PID, pregnancy,
and endometriosis can cause elevated CA125 levels in premenopausal
women; thus, measurement of CA125 levels is not as useful in
premenopausal women with adnexal masses. Values greater than 200 in a
premenopausal female may warrant gynecologic-oncology comanagement or
referral (81). Ultrasonographic characteristics are more helpful than CA125
in suggesting risks of malignancy in premenopausal women (84).
Imaging Studies
Other studies may be necessary or appropriate. [4] The most commonly indicated
study is pelvic ultrasonography, which will help document the origin of the mass
to determine whether it is uterine, adnexal, bowel, or gastrointestinal. The
ultrasonographic examination provides information about the size of the mass and
its consistency—unilocular cyst, mixed echogenicity, multiloculated cyst, or solid
mass—which can help determine management (Figs. 10-6 and 10-7). Size greater
than 10 cm, solid components, irregularity, papillary excrescences, and ascites
increase the suspicion of malignancy (81). A number of different ultrasound
scoring systems were developed in an effort to quantify the risks of malignancy.
FIGURE 10-6 Transvaginal ultrasound of a unilocular ovarian cyst.
FIGURE 10-7 Transvaginal ultrasonogram of a complex, predominantly solid mass.
[4] Transvaginal and transabdominal ultrasonography are complementary in the
diagnosis of pelvic masses, particularly those that have an abdominal component.
Transvaginal ultrasonography has the advantage of providing additional
information about the internal architecture or anatomy of the mass.
Heterogeneous pelvic masses, described as tubo-ovarian abscesses on
transabdominal ultrasonography, can be differentiated as pyosalpinx,
hydrosalpinx, tubo-ovarian complex, and tubo-ovarian abscess with transvaginal
ultrasonography (Fig. 10-8).
The diagnostic accuracy of transvaginal ultrasonography in diagnosing
endometrioma can be quite high (Fig. 10-9). Endometriomas can have a variety
of ultrasonographic appearances, from purely cystic to varying degrees of
complexity with septation or debris to a solid appearance. A variety of scoring
systems were developed with the intent of predicting benign versus malignant
adnexal masses using ultrasound; the ultrasonographic morphologic
characteristics used in many types of scoring systems are listed in Table 10-10
(81). Color flow Doppler was added to other sonographic characteristics to
predict the risk of malignancy; ultrasound techniques are comparable to CT and
MRI in differentiating benign from malignant masses (81,85). Although an
495analysis of such features may be helpful, histologic confirmation of surgically
removed persistent masses remains the standard of care.
FIGURE 10-8 Transvaginal ultrasonogram of bilateral tubo-ovarian abscesses.
FIGURE 10-9 Transvaginal ultrasonogram of an endometrioma of the ovary.
CT seldom is indicated as a primary diagnostic procedure, although it may be
helpful in planning treatment when a malignancy is strongly suspected or when a
nongynecologic disorder may be present. Abdominal flat-plate radiography is not
a primary diagnostic procedure, although if used for other indications, it may
reveal calcifications that can assist in the discovery or diagnosis of a mass. Pelvic
calcifications (teeth) consistent with a benign cystic teratoma, a calcified uterine
fibroid, or scattered calcifications consistent with psammoma bodies of a
papillary serous cystadenoma can be seen with abdominal radiography (Fig. 10-
10).
FIGURE 10-10 Benign cystic teratoma (dermoid cyst) of the ovary with teeth seen on
abdominal radiograph.
Ultrasonography or CT imaging may be appropriate to demonstrate ureteral
deviation, compression, or dilation in the presence of moderately large and
laterally located fibroids or other pelvic mass. Such findings rarely provide an
indication for surgical intervention for otherwise asymptomatic leiomyomas.
Table 10-10 Ultrasonographic Characteristics of Adnexal Masses That May Be Useful
in Predicting Malignancy
Unilocular cyst vs. multilocular vs. solid components
Regular contour vs. irregular border
Smooth walls vs. nodular vs. irregular
Presence or absence of ascites
Unilateral vs. bilateral
498Wall thickness
Internal echogenicity and septations (including thickness)
Presence of other intra-abdominal pathology (liver, etc.)
Vascular characteristics and color flow Doppler pattern
Hysteroscopy provides direct evidence of intrauterine pathology or
submucous leiomyomas that distort the uterine cavity (see Chapter 26).
Hysterosalpingography will demonstrate indirectly the contour of the endometrial
cavity and any distortion or obstruction of the uterotubal junction secondary to
leiomyomas, an extrinsic mass, or peritubal adhesions. The techniques combining
hysterosalpingography, in which fluid is instilled into the uterine cavity, with
transvaginal ultrasonography are helpful in the diagnosis of intrauterine
pathology. Hysterosalpingography or sonohysterography may be indicated in
women with infertility and uterine leiomyoma.
MRI may be most useful in the diagnosis of uterine anomalies, although its
value rarely justifies the increased cost of the procedure over ultrasonography for
the diagnosis of other pelvic masses (86).
Management of Pelvic Mass
The management of a pelvic mass is based on an accurate diagnosis. An
explanation of this diagnosis should be conveyed to the patient, along with a
discussion of the likely course of the disease (e.g., growth of uterine leiomyomas,
regression of fibroids at menopause, regression of a follicular cyst, the uncertain
malignant potential of an ovarian mass). All options for management should be
presented and discussed, although it is appropriate for the physician to state a
recommended approach with an explanation of the reasons for the
recommendation. Management should be based on the primary symptoms and
may include observation with close follow-up, temporizing surgical therapies,
medical management, or definitive surgical procedures.
Leiomyomas
[5] The management of uterine leiomyomas is dependent on the patient’s age and
proximity to anticipated menopause, symptoms, patient preference, and the
experience and skills of the clinician. Variability in reporting data regarding the
severity of symptoms, uterine anatomy, and response to therapy makes it difficult
to compare different types of therapies, which include observation, medical,
surgical, and radiologic-based techniques (see Chapter 11 for discussion of
uterine fibroids).
499Ovarian Masses
The now-routine application of ultrasound technology to gynecologic
examinations led to the more frequent detection of ovarian cysts, sometimes as an
incidental finding. Ultrasonography is a relatively easy diagnostic study to
perform, but this ease led to the labeling of physiologic ovarian morphology and
cystic follicles, as pathologic and the subsequent referral of patients for therapies,
including surgery, without indications. Treatment of ovarian masses that are
suspected to be functional tumors is expectant (Fig. 10-2). A number of
randomized prospective studies showed no acceleration of the resolution of
functional ovarian cysts (some of which were associated with the use of
clomiphene citrate or human menopausal gonadotropins) with oral
contraceptives compared with observation alone (66). Oral contraceptives
are effective in reducing the risk of subsequent ovarian cysts and may be
appropriate for women who desire both contraception and their
noncontraceptive benefits.
Symptomatic cysts should be evaluated promptly, although mildly
symptomatic masses suspected to be functional should be managed with
analgesics rather than surgery to avoid the risk of surgical complications,
including the development of adhesions that may impair subsequent fertility.
Surgical intervention is warranted in the presence of severe pain or the suspicion
of malignancy or torsion.
[4] On ultrasonography, ascites, cysts greater than 10 cm and those that have
multiloculations are concerning for malignancy (81). If a malignant mass is
suspected at any age, surgical evaluation should be performed promptly.
Simple cysts up to 10 cm in size are likely benign and can be managed
expectantly at any age, if asymptomatic (81).
Ovarian or adnexal torsion is suspected on the basis of peritoneal signs and the
acuity of onset, often accompanied by nausea and vomiting. Doppler flow studies
suggesting abnormal flow are predictive of torsion, although torsion can be seen
with normal flow (87). The absence of internal ovarian flow is not specific to
torsion and may be seen with cystic lesions, although in these situations
peripheral flow usually can be visualized.
The management of suspected ovarian torsion, which can occur at any age
from prepubertal to postmenopausal, is surgical. When torsion is confirmed
by laparoscopy, untwisting of the mass and ovarian preservation rather than
extirpation are generally indicated (88). The value of oophoropexy in
preventing recurrent torsion is not well established.
Ultrasonographic or CT-directed aspiration procedures of ovarian masses
should not be used in women in whom there is a suspicion of malignancy. In
the past, laparoscopic surgery for ovarian masses was reserved for diagnostic or
500therapeutic purposes in patients at very low risk for malignancy. With the recent
advancements in minimally invasive surgery the current recommendation is for
laparoscopic management of suspected benign adnexal masses (Fig. 10-11),
even those greater than 10 cm. Rates of intraoperative rupture were found to be
similar between the open and laparoscopic approaches in three randomized trials
made up of 394 patients. The benefits of laparoscopy included decreased
operative time, hospital stay, postoperative pain, and perioperative morbidity. The
conversion rate to laparotomy was less than 2% (81).
FIGURE 10-11 Laparoscopic appearance of benign ovarian mass (dermoid cyst).
Vulvar Conditions
In postmenarchal individuals, vulvar symptoms are most often related to a
primary vaginitis and a secondary vulvitis. The mere presence of vaginal
discharge can lead to vulvar irritative symptoms, or candidal vulvitis may be
present (Fig. 10-12). The causes of vaginitis and cervicitis are covered in Chapter
15. Adult women describe vulvar symptoms using a variety of terms (itching,
pain, discharge, discomfort, burning, external dysuria, soreness, pain with
501intercourse or sexual activity). Burning with urination from noninfectious
causes may be difficult to distinguish from a urinary tract infection, although
some women can distinguish pain when the urine hits the vulvar area (an
external dysuria) from burning pain (often suprapubic in location) during
urination. Itching is a very common vulvar symptom. A variety of vulvar
conditions and lesions can present with pruritus. Vulvovaginal symptoms may be
caused by STDs, nonsexually transmitted vaginitis, or UTIs. The distinction
between symptoms related to a UTI and those of vaginitis is difficult, and
consideration should be given to testing for both C. trachomatis and obtaining a
urine culture, particularly in young reproductive age women (89).
FIGURE 10-12 Candidal vulvitis.
A number of skin conditions that occur on other areas of the body may occur
on the vulvar area. Table 10-11 contains a list of these conditions classified by
either infectious or noninfectious causes. Whereas the diagnosis of some of these
conditions is apparent from inspection alone (e.g., a skin tag), any lesions that
appear atypical or in which the diagnosis is not clear should be analyzed by
502biopsy, because the risks of malignant lesions increases with age (Fig. 10-13).
Pigmented vulvar lesions include benign nevi, lentigines, melanosis, seborrheic
keratosis, condyloma, and some vulvar intraepithelial neoplasias (VINs),
especially multifocal VIN-3 (Fig. 10-14). Suspicious pigmented vulvar lesions
in particular should warrant biopsy to rule out VIN or malignant melanoma
(90). Approximately 10% of white women have a pigmented vulvar lesion; some
of these lesions may be malignant (see Chapter 40) or have the potential for
progression (VIN) (see Chapter 16). There is an increase in rates of VIN in
women younger than age 50, along with increasing rates of vulvar squamous cell
carcinoma in situ, possibly related to increasing rates of human papillomavirus
(HPV) infection. Heightened awareness among clinicians may play a role in the
increasing frequency of diagnosis; suspicious lesions warrant vulvar biopsy.
Pigmented lesions include common nevi, lentigines, melanomas, dysplastic nevi,
blue nevi, and a lesion termed atypical melanocytic nevi of the genital type
(AMNGT) (91). AMNGTs have some histologic features that may overlap with
those of melanoma, but with a benign prognosis.
Table 10-11 Subacute and Chronic Skin Recurrent Conditions of the Vulva
Noninfectious Infectious
Acanthosis nigricans Cellulitis
Atopic dermatitis Folliculitis
Behçet disease Furuncle/carbuncle
Contact dermatitis Insect bites (e.g., chiggers, fleas)
Crohn disease Necrotizing fasciitis
Diabetic vulvitisa Pubic lice
Hidradenitis suppurativaa Scabies
Tinea
Lichen sclerosus Condyloma
Paget disease Vulvar candidiasis
“Razor bumps”—folliculitis or pseudofolliculitis HSV
Psoriasis
503Seborrheic dermatitis
Vulvar aphthous ulcer
Vulvar intraepithelial neoplasia
aEtiology unknown, often secondarily infected.
FIGURE 10-13 Large benign skin tag from left labium majus.
FIGURE 10-14 Pigmented vulvar lesion.
Vulvar Biopsy
A vulvar biopsy is essential for distinguishing benign from premalignant or
malignant vulvar lesions, especially because many types of lesions may have
a somewhat similar appearance. Vulvar biopsies should be performed liberally
in women of reproductive age to ensure that these lesions are diagnosed and
treated appropriately. A prospective study of vulvar lesions evaluated by biopsy
in a gynecologic clinic found lesions occurring in the following order of
frequency: epidermal inclusion cyst, lentigo, Bartholin duct obstruction,
carcinoma in situ, melanocytic nevi, acrochordon, mucous cyst, hemangiomas,
postinflammatory hyperpigmentation, seborrheic keratoses, varicosities,
hidradenomas, verruca, basal cell carcinoma, and unusual tumors such as
neurofibromas, ectopic tissue, syringomas, and abscesses (92). The frequency
with which a lesion would be reported after a tissue biopsy is related to the
frequency with which all lesions of a given pathology are evaluated in this
manner. This listing probably underrepresents such common lesions as
condylomata (Fig. 10-15).
FIGURE 10-15 Extensive vulvar condyloma.
Biopsy is easily performed in the office using a local anesthetic. Typically,
1% lidocaine is infiltrated beneath the lesion using a small (25- to 27-gauge)
needle. Disposable punch biopsy instruments come in a variety of sizes from 2 to
6 mm in diameter. These skin biopsy instruments, along with fine forceps,
scissors, and a scalpel, should be available in all outpatient gynecologic settings.
For smaller biopsies, it is usually not necessary to place a suture. Topical silver
nitrate can be used for hemostasis. Multiple tissue samples may be appropriate to
obtain representative areas of a lesion if the lesion has a variable appearance or is
multifocal. Although the vulvar biopsy procedure involves minimal discomfort
during the procedure, the biopsy sites will be painful for several days after the
procedure. The prescription of a topical anesthetic such as 2% lidocaine jelly, to
be applied periodically and before urinating, is appreciated by patients who
require this procedure. Infection of the site can occur, and patients should be
cautioned to report excessive erythema or purulent drainage.
Other Vulvar Conditions
506Classification and description of intraepithelial lesions of the vulva are presented
in Chapter 16.
Pseudofolliculitis or Mechanical Folliculitis
This is similar to what is described as pseudofolliculitis barbae (razor bumps) and
may occur in women who follow the popular practice of shaving pubic hair (93).
Pseudofolliculitis consists of an inflammatory reaction surrounding an
ingrown hair and occurs most commonly among individuals with curly hair,
particularly African Americans.
Infectious Folliculitis
Shaving may be associated with an infectious folliculitis, commonly caused by
Staphylococcus aureus and Streptococcus pyogenes. Shaving and other methods
of pubic hair removal are associated with razor burn, contact dermatitis, and the
transmission of other infectious agents such as Molluscum contagiosum, HPV,
and herpes simplex along with other bacteria including Pseudomonas aeruginosa
(93).
Fox–Fordyce Disease
This condition is characterized by a chronic, pruritic eruption of small papules or
cysts formed by keratin-plugged apocrine glands. It is commonly present over the
lower abdomen, mons pubis, labia majora, and inner portions of the thighs.
Hidradenitis suppurativa is a chronic condition involving the apocrine
glands with the formation of multiple deep nodules, scars, pits, and sinuses
that occur in the axilla, vulva, and perineum. Hyperpigmentation and
secondary infection are often seen. Hidradenitis suppurativa can be extremely
painful and debilitating. It has been treated with antibiotics, isotretinoin, or
steroids; surgical therapy with wide local excision may be necessary (94).
Acanthosis Nigricans
This disease involves widespread velvety pigmentation in skin folds, particularly
the axillae, neck, thighs, submammary area, and vulva and surrounding skin (Fig.
10-16). It is of particular interest to gynecologists because of its association with
hyperandrogenism and PCOS; as such, it is associated with obesity, chronic
anovulation, acne, glucose intolerance, insulin resistance, and cardiovascular
disease (95). Topical and oral retinoids are used to treat the acanthosis nigricans,
along with management of the underlying conditions including obesity and
insulin resistance or diabetes.
FIGURE 10-16 Acanthosis nigricans of the neck.
Extramammary Paget Disease
This is an intraepithelial neoplasia containing vacuolated Paget cells (see Chapter
16). Clinically, the appearance of Paget disease is variable, and it may have an
appearance varying from moist, oozing ulcerations to an eczematoid lesion with
scaling and crusting to a grayish lesion (96). It may be confused with candidiasis,
psoriasis, seborrheic dermatitis, contact dermatitis, and VIN. A biopsy to confirm
the diagnosis is mandatory. Treatment has traditionally been surgery, although
recurrences are very common; topical therapies including imiquimod are being
used (97).
Vulvar Intraepithelial Neoplasia
VIN is associated with HPV infection and is increasing in frequency,
particularly among young women (see Chapter 16). Diagnosis requires biopsy
of any suspicious vulvar lesions, particularly those that are pigmented or
discolored. The increasing frequency of this entity dictates a careful vulvar
inspection during annual gynecologic examinations.
508Vulvar Tumors, Cysts, and Masses
Condylomata Acuminata
These are very common vulvar lesions and are usually easily recognized.
They may resolve spontaneously; treatment is guided by wart number, size,
and anatomic site; patient preference; cost of treatment; convenience;
adverse effects; and provider experience (98). Treatments may be patientapplied or provider-administered. Other sexually transmitted organisms,
such as the virus responsible for M. contagiosum and the lesions of syphilis
and condylomata lata, may occasionally be mistaken for vulvar condylomata
acuminata caused by HPV (see Chapter 15). A summary of benign vulvar
tumors is listed in Table 10-12. There is an argument regarding whether
sebaceous cysts exist on the vulva or whether these lesions are histopathologically
epidermal or epidermal inclusion cysts (99). The so-called sebaceous cysts are
clinically indistinguishable from epidermal inclusion cysts that may result from
the burial of fragments of skin after the trauma of childbirth or episiotomy or that
arise from occluded pilosebaceous ducts. These cysts are seldom symptomatic,
although if infection develops, incision and drainage may be required acutely, and
ultimately complete excision is indicated.
Table 10-12 Types of Vulvar Tumors
1. Cystic lesions
Bartholin duct cyst
Cyst in the canal of Nuck (hydrocele)
Epithelial inclusion cyst
Skene duct cyst
2. Solid tumors
Acrochordon (skin tag)
Angiokeratoma
Bartholin gland adenoma
Cherry angioma
Fibroma
509Hemangioma
Hidradenoma
Lipoma
Granular cell myoblastoma
Neurofibroma
Papillomatosis
3. Anatomic
Hernia
Urethral diverticulum
Varicosities
4. Infections
Abscess—Bartholin, Skene, periclitoral, other
Condyloma lata
Molluscum contagiosum
Pyogenic granuloma
5. Ectopic
Endometriosis
Ectopic breast tissue
Bartholin Duct Cysts
These are common vulvar lesions in reproductive age women. They result from
occlusion of the duct with accumulation of mucus and may be asymptomatic.
Infection of the gland can result in the accumulation of purulent material, with the
formation of a rapidly enlarging, painful, inflammatory mass (a Bartholin
abscess). An inflatable bulb-tipped catheter was described by Word and is quite
easy to use (100). The small catheter is inserted through a small stab wound into
the abscess after infiltration of the skin with local anesthesia; the balloon of the
catheter is inflated with 2 to 3 mL of saline and the catheter remains in place for 4
to 6 weeks, allowing epithelialization of a tract and the creation of a permanent
510gland opening.
Skene Duct Cysts
These are cystic dilations of the Skene glands, typically located adjacent to the
urethral meatus within the vulvar vestibule. Although most are small and often
asymptomatic, they may enlarge and cause urinary obstruction, requiring excision
(Fig. 10-17).
FIGURE 10-17 The Skene gland cyst.
Painful Intercourse
Painful intercourse (dyspareunia) may be caused by many different vulvovaginal
conditions, including common vaginal infections and vaginismus (see Chapters
15 and 17). A careful sexual history is essential, as is a careful examination of the
vulvar area and vagina. Vulvodynia is the term used to describe unexplained
vulvar pain, sexual dysfunction, and the resultant psychological disability
(101,102). The term vulvar vestibulitis was previously used to describe a
situation in which there is pain during intercourse or when attempting to
511insert an object into the vagina; pain on pressure to the vestibule on
examination and vestibular erythema (known as the Friedrich triad); this
entity is now described as localized vulvodynia, which may be provoked or
spontaneous, primary or secondary, and intermittent, persistent, constant,
immediate, or delayed (see Chapter 12) (102,103). A number of studies failed to
demonstrate a consistent relationship with any genital infectious organism,
including C. trachomatis, gonorrhea, Trichomonas, mycoplasma, Ureaplasma,
Gardnerella, candida, or HPV, and the condition has been characterized as
multifactorial, with inflammatory, neuropathic, and functional components (104).
Although the symptoms of dyspareunia with insertion can be disabling, no
curative therapies were found. Medical and behavioral therapies are of some
benefit, and some authors encourage surgery, but the role of this treatment and
newer therapies such as the injection of botulinum toxin A is not well established
(104).
Vulvar Ulcers
A number of STDs can cause vulvar ulcers, including herpes simplex virus,
syphilis, lymphogranuloma venereum, and granuloma inguinale (see Chapter 15).
Crohn disease can include vulvar involvement with abscesses, fistulae, sinus
tracts, fenestrations, and other scarring. Medical treatment with systemic steroids
and other systemic agents is the standard therapy; surgical therapy for intestinal
and vulvar disease may be required.
The Behçet Disease
This systemic condition is characterized by genital and oral ulcerations with
ocular inflammation and many other manifestations (105). The cause and the
most effective therapy are not well established, although anti-inflammatory,
immunomodular, and immunosuppressive therapies may be effective (106).
Lichen Planus
This condition causes oral and genital ulcerations. Typically, there is
desquamative vaginitis with erosion of the vestibule. Treatment is based on the
use of topical and systemic steroids. Plasma cell mucositis appears as erosions in
the vulvar area, particularly the vestibule. Biopsy is essential in establishing the
diagnosis.
Vaginal Conditions
Vaginal discharge is one of the most common vaginal symptoms. Conditions
ranging from vaginal candidiasis to chlamydia cervicitis to bacterial vaginosis to
cervical carcinoma may cause vaginal discharge. Infectious vaginal conditions are
512addressed more completely in Chapter 15. Vaginal lesions may occasionally be
palpable to a woman. More commonly, vaginal lesions are discovered on
examination by a clinician. They may contribute to symptoms (such as bleeding
or discharge) or they may be entirely asymptomatic. Vaginitis, cervicitis, and
vaginal or cervical lesions (including malignancies) can be causes of vaginal
discharge. Other noninfectious causes of discharge are as follows:
1. Retained foreign body—tampon, pessary
2. Ulcerations—tampon-induced, lichen planus, herpes simplex infection
3. Malignancy—cervical, vaginal
Some vaginal lesions are asymptomatic and are noted incidentally on
examination. Fibroepithelial polyps consist of polypoid folds of connective tissue,
capillaries, and stroma covered by vaginal epithelium. Although they can be
excised easily in the office, their vascularity can be troublesome, and excision is
not necessary unless the diagnosis is in question. Cysts of embryonic origin can
arise from mesonephric, paramesonephric, and urogenital sinus epithelium.
Gartner duct cysts are of mesonephric origin and are usually present on the
lateral vaginal wall. They rarely cause symptoms and do not require treatment.
Other embryonic cysts can arise anterior to the vagina and beneath the bladder.
Cysts that arise from the urogenital sinus epithelium are located in the area of the
vulvar vestibule. Vaginal adenosis, the presence of epithelial-lined glands within
the vagina, is associated with in utero exposure to diethylstilbestrol. No therapy is
necessary other than close observation and periodic palpation to detect nodules
that may need to be evaluated by biopsy to rule out vaginal clear cell
adenocarcinoma (see Chapter 38).
Women will sometimes describe a bulging lesion of the vagina and vulvar area,
variably associated with symptoms of pressure or discomfort. The most common
cause of such a lesion is one of the disorders of vaginal support. Management of
these conditions is discussed in Chapter 30. Other genital lesions, such as urethral
diverticula or embryonic cysts, may cause similar symptoms.
POSTMENOPAUSAL AGE GROUP
Abnormal Bleeding
Differential Diagnosis
[1] The causes of postmenopausal bleeding and the percentage of patients who
seek treatment for different conditions are presented in Table 10-13.
Table 10-13 Etiology of Postmenopausal Bleeding
513Factor Approximate
Percentage
Exogenous estrogens 30
Atrophic endometritis/vaginitis 30
Endometrial cancer 15
Endometrial or cervical polyps 10
Endometrial hyperplasia 5
Miscellaneous (e.g., cervical cancer, uterine sarcoma, urethral
caruncle, trauma)
10
From Hacker NF, Moore JG. Essentials of Obstetrics and Gynecology. 3rd ed.
Philadelphia, PA: WB Saunders; 1998:635, with permission.
Benign Disorders
Hormone therapy (HT) may be used to manage troublesome menopausal
symptoms including vasomotor and genitourinary symptoms. The North
American Menopause Society has issued a statement noting that HT is the most
effective treatment for these symptoms, and has been shown to prevent bone loss
and fracture; the society noted that the risks of HT depend on the type, dose,
duration, route of administration, timing of initiation, and concurrent use of a
progestogen, thus requiring individualization of use, with periodic revaluation of
the benefits versus the risks (107). Long-term combined HT has been associated
with increased risks of venous thromboembolism, stroke, breast cancer, coronary
events, gallbladder disease, and death from lung cancer (108). Women who are
taking HT during menopause may be using a variety of hormonal regimens that
can result in bleeding. Because unopposed estrogen therapy can result in
endometrial hyperplasia, various regimens of progestins are typically added to the
estrogen regimen; they are given in a continuous fashion, although they may be
given in a sequential fashion for women within 1 year of menopause. Endometrial
sampling is indicated for any unexpected bleeding that occurs with hormonal
therapy. A significant change in withdrawal bleeding or breakthrough
bleeding (e.g., absence of withdrawal bleeding for several months followed by
resumption of bleeding or a marked increase in the amount of bleeding)
should prompt endometrial sampling.
Patient adherence to hormonal regimens is a significant issue with HT, with the
challenges of oral therapy mitigated by nonoral routes of administration (109).
514Missed doses of oral medication and failure to take the medication in the
prescribed fashion can lead to irregular bleeding or spotting that is benign in
origin but that can result in patient dissatisfaction.
The problems that women most often report with HT include vaginal bleeding
and weight gain. The use of a continuous low-dose combined regimen has the
advantage that for many women, bleeding will ultimately cease after several
months, during which irregular and unpredictable bleeding may occur (110).
Some women are unable to tolerate these initial months of irregular bleeding. The
risk of endometrial hyperplasia or neoplasia with this regimen is low.
Other benign causes of bleeding include atrophic vaginitis and endometrial and
cervical polyps, which may become apparent as postcoital bleeding or spotting.
Women who experience bleeding after menopause may attempt to minimize the
extent of the problem; they may describe it as “spotting” or “pink or brownish
discharge.” However, any indication of bleeding or spotting should be evaluated.
[1] In the absence of HT, any bleeding after menopause (classically defined
as absence of menses for 1 year) should prompt evaluation with endometrial
sampling. Studies of transvaginal ultrasonography revealing an endometrial
thickness 4 mm or less correlate with a low risk of endometrial malignancy, and
thus endometrial sampling is not required (111). Endometrial polyps and other
abnormalities can be seen in women who are taking tamoxifen. These polyps are
more likely to involve cystic dilation of glands, stromal condensation around the
glands, and squamous metaplasia of the overlying epithelium. These polyps can
be benign, although they must be distinguished from endometrial malignancies,
which may occur when taking tamoxifen. [2] The incidence of endometrial polyps
not associated with tamoxifen increases with age during the reproductive years; it
is not clear whether the incidence subsequently peaks or decreases during the
postmenopausal years (12). Endometrial polyps are more likely to be malignant in
postmenopausal women, and hypertension is associated with an increased risk of
malignancy (112).
Neoplasia
Endometrial, cervical, and ovarian malignancies must be ruled out in the presence
of postmenopausal bleeding. One series found a malignancy (endometrial or
cervical) in approximately 10% of women with postmenopausal bleeding (113).
Cervical cytology testing is essential when postmenopausal bleeding is noted,
although cytologic testing is an insensitive diagnostic tool for detecting
endometrial cancer. The cytology test results are negative in some cases of
invasive cervical carcinoma because of tumor necrosis.
Cervical malignancy is diagnosed by cervical biopsy of grossly visible
lesions and colposcopically directed biopsy for women with abnormal Pap
515test results (see Chapter 16). Functional ovarian tumors may produce
estrogen and lead to endometrial hyperplasia or carcinoma, which may cause
bleeding.
Diagnosis of Postmenopausal Abnormal Bleeding
Pelvic examination to detect local lesions and a Pap test to assess the cytology are
essential first steps in finding the cause of postmenopausal bleeding. Pelvic
ultrasonographic examination and, in particular, transvaginal ultrasonography or
sonohysterography can suggest the cause of bleeding (111,114). Endometrial
sampling, through office biopsy, hysteroscopy, or D&C, is usually considered
essential. An endometrial thickness of less than 5 mm measured by transvaginal
ultrasonography is unlikely to indicate endometrial cancer, and some authors
suggest that the diagnostic accuracy is overestimated and recommend a cutoff of
3 mm (115).
Management of Postmenopausal Abnormal Bleeding
Benign Disorders
The management of bleeding caused by atrophic vaginitis includes topical
(vaginal) or systemic use of estrogens after other causes of abnormal bleeding are
excluded. Such therapy can provide significant benefits in terms of the quality of
life, but must be weighed with each individual, considering contraindications and
patient preferences (107). Serum levels appear to be lower with vaginal
administration using creams, tablets, or rings (116). Cervical polyps can easily be
removed in the office.
Endometrial Hyperplasia
The terminology used to describe endometrial hyperplasia is confusing, and the
clinician must consult with the pathologist to ensure an understanding of the
diagnosis to dictate correct management. In 2015, the ACOG and the Society of
Gynecologic Oncology recommended that the World Health Organization
(WHO) system be replaced with the endometrial intraepithelial neoplasia
(EIN) schema as described in Table 10-14 to distinguish benign lesions from
those that are premalignant and should be managed more conservatively.
Table 10-14 Nomenclature for Endometrial Hyperplasia
Class Nomenclature Treatment
Benign Benign endometrial hyperplasia Medical
516Premalignant Endometrial intraepithelial
neoplasia (EIN), replaces atypical
hyperplasia
Surgical vs. medical (desires
fertility or poor surgical
candidate)
Malignant Endometrial adenocarcinoma,
endometrioid type, well
differentiated
Surgical +/– staging
The new nomenclature has three categories:
1. Benign = Benign endometrial hyperplasia
2. Premalignant = EIN, replaces atypical hyperplasia
3. Malignant = Endometrial adenocarcinoma, endometrioid type, well
differentiated
Many pathologists still use the WHO system, which classifies endometrial
hyperplasia as simple hyperplasia, complex hyperplasia, simple atypical
hyperplasia, and complex atypical hyperplasia (117). Approximately 40% to
50% of women with atypical hyperplasia or EIN have concurrent carcinoma. The
management of endometrial hyperplasia is based on an understanding of the
natural history of the lesion involved. The risk of progression of hyperplasia
without atypia is low but is approximately 30% among those with atypical
hyperplasia (117). Hysterectomy is recommended as definitive treatment of EIN
in postmenopausal women, and rules out possible concurrent carcinoma.
Management of endometrial cancer with surgical staging and multidisciplinary
review of pathology and treatment planning is addressed in Chapter 37.
Progestin therapy (oral, parenteral, or intrauterine device delivery) has been
used in women with EIN or endometrial cancer who are poor operative candidates
(29,118). These women should have an endometrial biopsy every 3 months to
check for recurrence, with recurrence risks approaching 50% (118). A suggested
scheme of management is outlined in Figure 10-18. This treatment is discussed in
more detail in Chapter 37.
FIGURE 10-18 Management of endometrial hyperplasia. (From Hacker NF, Friedlander
ML. Uterine cancer. In: Berek JS, Hacker NF. Berek & Hacker’s Gynecologic Oncology.
6th ed. Philadelphia, PA: Wolters Kluwer; 2014: 404.)
Pelvic Masses
Differential Diagnosis
Ovarian Masses
During the postmenopausal years, the ovaries become smaller. Ovarian
volume is related to age, menopausal status, weight, height, and the use of
exogenous hormones (119). A large body habitus and uterine size make it more
difficult to palpate and assess the ovarian size, particularly among
postmenopausal women, and transvaginal ultrasonography is significantly more
accurate than clinical examination. Transvaginal ultrasonography is suggested in
addition to annual pelvic examination among overweight postmenopausal women
(120). [3] Although ovarian cancer is notoriously difficult to diagnose at any early
stage, the concept that it is frequently asymptomatic has been challenged.
Symptoms may include back pain, fatigue, bloating, constipation, abdominal pain,
and urinary symptoms; these symptoms are of greater severity and more recent
onset in women with ovarian malignancy (121). It is argued that among primary
clinicians, the possibility of an ovarian mass (either benign or malignant) in
women with these symptoms warrants further diagnostic investigation. The
positive predictive value of these symptoms is not high for the prediction of earlystage disease, and the use of symptoms to trigger an evaluation for ovarian cancer
is noted to result in diagnosis of the disease in only 1 in 100 women in the general
population with such symptoms (122). [3] Ovarian cancer is predominantly a
disease of postmenopausal women; the incidence increases with age, and the
average patient age is about 56 to 60 years (see Chapter 39).
With increased use of pelvic ultrasonographic evaluation, a new problem
arose in postmenopausal women: the discovery of a small ovarian cyst. This
is particularly troublesome in a woman who is entirely asymptomatic and whose
ultrasonographic examination was performed for indications unrelated to pelvic
pathology. It is suggested that when the cyst is asymptomatic, small (<10 cm in
diameter), unilocular, and thin walled, with a normal CA125 level, the risk of
malignancy is extremely low and these masses can be followed
conservatively, without surgery (81,123,124). Surgery may be indicated in
some women with a strong family history of ovarian, breast, endometrial, or
colon cancer, or with a mass that appears to be enlarging (see Chapter 39). The
addition of color flow Doppler examination and other ultrasonographic
519characteristics may be helpful in distinguishing benign from malignant masses,
although the role of Doppler ultrasonography remains somewhat controversial
(Table 10-10) (81).
Uterine and Other Masses
[5] Many postmenopausal women have not had regular gynecologic care, and the
discovery of a pelvic mass may reflect the persistence of a uterine leiomyoma that
previously was not discovered. The possibility of transient ovarian cysts is noted
above, and it may be difficult to distinguish an ovarian from a uterine mass. Some
women may not remember having been told they had a pelvic mass. Thus, a
review of medical records may be helpful in determining the pre-existence of
a benign pelvic mass. Uterine leiomyomas are hormonally responsive and
typically decrease in size or resolve after menopause (see Chapter 11).
Diagnosis of Postmenopausal Pelvic Mass
A personal and family medical history is helpful in detecting individuals at
increased risk for the development of ovarian cancer. Several hereditary
family cancer syndromes involve ovarian neoplasms (see Chapter 39). Patients
with hereditary forms of epithelial ovarian cancer account for only a small
percentage of all cases; 90% to 95% of cases of ovarian cancer are sporadic
and without identifiable heritable risk.
In postmenopausal women with a pelvic mass, a CA125 measurement may be
helpful in predicting a higher likelihood of a malignancy, which may guide
decisions regarding management, consultation, or referral. A high index of
suspicion by women and their clinicians represents the best way to detect early
ovarian cancer. Persistent symptoms such as an increase in abdominal size,
bloating, fatigue, abdominal pain, indigestion, inability to eat normally, urinary
frequency, pelvic pain, constipation, back pain, new onset of urinary
incontinence, or unexplained weight loss require evaluation and consideration of
the possibility of ovarian cancer. A normal CA125 level does not rule out
ovarian cancer; up to 50% of early-stage ovarian malignancies and 20% to
25% of advanced cancers have normal values of CA125 (125).
Management of Postmenopausal Pelvic Mass
The use of improved imaging techniques may allow the nonoperative
management of ovarian masses that are probably benign (Table 10-10). A
suspicious or persistent complex mass requires surgical evaluation. Patients
with masses that are clinically suspicious for cancer should be offered the
opportunity of a preoperative consultation with a gynecologic oncologist, a
520physician trained to appropriately stage and debulk ovarian cancer to optimize the
patient’s outcome (126). Comprehensive surgical staging facilitates appropriate
therapy and optimizes prognosis.
Vulvar Conditions
Anatomic changes that occur in postmenopausal women include atrophy of the
labia majora and increasing prominence of the labia minora. The epithelium of the
hymen and vestibule become thin; there is a shift in vaginal cellular maturation in
response to estrogen deprivation, with resultant thinning. Although these changes
lead to minimal symptoms in most women, external dysuria, pruritus, tenderness,
dyspareunia, and bleeding can result from fissuring and excoriations. Because of
the risk of VIN and malignancy, suspicious lesions require vulvar biopsy.
Vulvar Dermatoses
Several vulvar conditions occur most commonly in postmenopausal women.
Symptoms are primarily itching and vulvar soreness, in addition to dyspareunia.
In the past, numerous terms were used to describe disorders of vulvar epithelial
growth that produce a number of nonspecific gross changes. These terms included
leukoplakia, lichen sclerosus, and atrophicus, atrophic and hyperplastic vulvitis,
and kraurosis vulvae. The ISSVD in 2006 recommended a classification of vulvar
dermatoses based on histologic patterns, listed with the likely clinical diagnoses,
rather than on the basis of clinical morphology as in the previous categorization
(see Chapter 12) (127). This classification system excludes neoplastic and
infectious conditions. Vulvar conditions that are described in this classification
system include atopic, allergic, and irritant contact dermatitis, psoriasis, lichen
simplex chronicus, lichen sclerosus, lichen planus, pemphigoid, aphthous ulcers,
Behçet disease, and Crohn disease.
Lichen Sclerosus
Lichen sclerosus is the most common white lesion of the vulva. Lichen
sclerosus can occur at any age, although it is most common among
postmenopausal women and prepubertal girls (Fig. 10-19). The symptoms are
pruritus, dyspareunia, and burning. Lichen sclerosus characteristically is
associated with decreased subcutaneous fat to the extent that the vulva is atrophic,
with small or absent labia minora, obliteration of the anatomic landmarks, thin
labia majora, and sometimes phimosis of the prepuce. The surface is pale with a
shiny, crinkled pattern (described as having characteristics like “cigarette paper”),
often with fissures and excoriation. The lesion tends to be symmetric and often
extends to the perineal and perianal areas. The diagnosis is confirmed by biopsy.
Invasive cancer is associated with lichen sclerosus, although the significance of
521this association is unclear in terms of causation (128).
FIGURE 10-19 Prepubertal lichen sclerosus.
523Treatment is with an ultrapotent topical steroid such as 0.05% clobetasol.
Approximately 96% of patients respond satisfactorily (129). Maintenance
therapy is frequently required, and a graduated reduction from ultrapotent to
medium- and low-potency topical steroids can help to maintain remission of
symptoms (130). The topical calcineurin inhibitors pimecrolimus and tacrolimus
were effective in individuals not responding to topical steroids, although the FDA
approved a warning suggesting possible risk of cancer from this class of drugs
and caution is advised with long-term use avoided (131).
Premalignant Vulvar Lesions
Squamous VIN is seen most often in postmenopausal women but may occur
during the reproductive years. Pruritus is the most common symptom, although
“lumps” may be described and are sometimes confused with condyloma (132).
Current terminology describes two types of VINs: VIN, usual type, that is
typically HPV-related and encompassing former VIN-2 and -3 with warty,
basaloid, and mixed types; and VIN, differentiated type (133). The lesion appears
thickened and hyperkeratotic, and there may be excoriation. Lesions may be
discrete but may be symmetric and multiple. About one-third of women will have
a history of HPV-related cervical disease or condyloma (132). Most women with
VIN are smokers. Biopsy is necessary to confirm the diagnosis and to exclude
malignancy. See Chapter 16 for management of VIN.
Urethral Lesions
The urethra and vagina have a common embryonic origin and are steroiddependent tissues. Urethral caruncles and prolapse of the urethral mucosa are
examples of vulvar lesions that may be seen in other age groups but that occur
more commonly among older women. Both conditions can be treated with topical
or systemic estrogen preparations. Various vulvar skin lesions, including
seborrheic keratoses and cherry hemangiomas (senile hemangiomas), occur more
commonly on aging skin.
Vaginal Conditions
Up to half of postmenopausal women have symptoms of atrophic vaginitis,
although many do not seek therapy. Symptoms include an external dysuria,
pruritus, tenderness, dyspareunia, and bleeding from fissuring or ulcerations. In
addition to the clinical findings of a shiny, flat, thin-appearing vaginal mucosa
without rugae, microscopic examination of vaginal secretions reveals an increased
number of white blood cells. Treatment with local or systemic estrogens
effectively manages the symptoms and restores normal pH levels with
ongoing therapy (134). Systemic absorption does occur with topical estrogen
524therapy, and rates of absorption differ depending on the degree of atrophy.
Topical emollients may be helpful if estrogens are not desired or are
contraindicated. Vaginal lubricants are universally useful in minimizing
symptoms of dyspareunia for postmenopausal women (135).





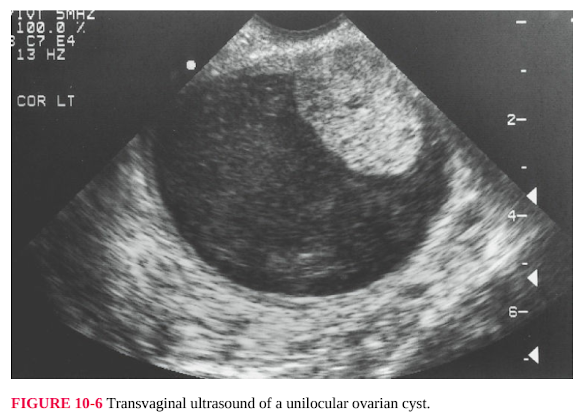








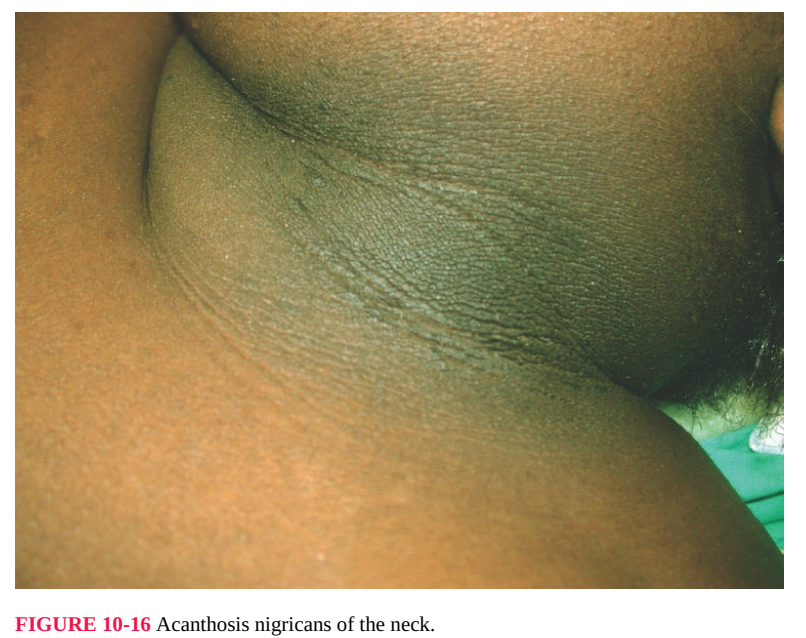



Nhận xét
Đăng nhận xét