Endometriosis
BS. Nguyễn Hồng Anh
KEY POINTS
1 Endometriosis is diagnosed by visualization of lesions during laparoscopy, ideally with histologic confirmation; positive histology confirms the diagnosis, but negative histology does not exclude it.
2 Endometriosis can be associated with infertility, pelvic pain, that is, dysmenorrhea, dyspareunia and nonmenstrual pain, and reduced quality of life.
3 Severe or deep endometriosis should be managed in a facility with the necessary expertise to provide treatment in a multidisciplinary context, including advanced laparoscopic surgery and laparotomy.
4 The American Society for Reproductive Medicine (ASRM) staging system for endometriosis is subjective and correlates poorly with pelvic pain and infertility.
5 The Endometriosis Fertility Index (EFI) predicts non-in vitro fertilization (IVF) pregnancy rates after surgical treatment of endometriosis.
6 Suppression of ovarian function reduces pain associated with endometriosis. Different classes of hormonal drugs—combination oral contraceptives, progestins, gonadotropin-releasing hormone (GnRH) agonists—are equally effective in reducing pain but have differing side effects and costs.
7 Ablation or resection of endometriotic lesions plus adhesiolysis in minimal to mild endometriosis is more effective than diagnostic laparoscopy alone in improving fertility.
8 Suppression of ovarian function is not effective in improving subsequent fertility in patients with endometriosis.
Endometriosis is defined as the presence of endometrial-like tissue (glands and/or stroma) outside the uterus. The most frequent sites of implantation are the pelvic viscera and the peritoneum but, although rare, it can also be found in the pericardium, pleura, lung, and even the brain. Endometriosis varies in appearance from a few minimal lesions on otherwise intact pelvic organs, to deep infiltrating nodules and massive ovarian endometriotic cysts with extensive adhesions involving bowel, bladder, and ureter resulting in significant distortion of the pelvic anatomy. It is estimated to occur in 10% of reproductiveage women and is associated with pelvic pain and infertility. Considerable progress in understanding the pathogenesis, spontaneous evolution, diagnosis, and treatment of endometriosis has occurred. The European Society for Human
Reproduction and Embryology (ESHRE) guidelines for the clinical management of endometriosis are published and regularly updated to present emerging clinical evidence (1).
EPIDEMIOLOGY
Prevalence
Endometriosis is found predominantly in women of reproductive age but is reported in adolescents and in postmenopausal women receiving hormonal replacement therapy (2). It is found in women of all ethnic and social groups.
Estimates of the frequency of endometriosis vary widely, but the prevalence of the condition is assumed to be around 10% women of reproductive age (3,4). Although no consistent information is available on the incidence of the disease, temporal trends suggest an increase among women of reproductive age (4). The World Endometriosis Research Foundation (WERF) EndoCost study calculated the costs of women with histologically proven endometriosis treated in referral centers (5). The study showed that the costs to women with endometriosis are substantial, resulting in an economic burden similar to the estimated annual health care costs for diabetes mellitus, Crohn disease, and rheumatoid arthritis (5).
In women with pelvic pain or infertility, a high prevalence of endometriosis (from a low of 20% to a high of 90%) is reported (6,7). In women with unexplained infertility (regular menstrual cycle, normal pelvic imaging, normospermic partner) with or without pain, the prevalence of endometriosis is reported to be as high as 50% (8). In asymptomatic women undergoing tubal ligation (women of proven fertility), the prevalence of endometriosis ranges from 3% to 43% (9–14). This variation in the reported prevalence may be explained by several factors.
First, it may vary with the diagnostic method used: laparoscopy, the operation of choice for diagnosis, is a better method than laparotomy for diagnosing minimal to mild endometriosis.
Second, minimal or mild endometriosis may be more thoroughly evaluated in asymptomatic patients than in asymptomatic patients during tubal sterilization.
Third, the interest and experience of the surgeon has relevance because there is a wide variation in the appearance of subtle endometriosis implants, cysts, and adhesions. Most studies that evaluate the prevalence of endometriosis in women of reproductive age lack histologic confirmation (9–11,15–20).
Risk and Protective Factors
The following are possible risk factors for endometriosis: infertility, red hair, early age at menarche, shorter menstrual cycle length, hypermenorrhea, nulliparity, müllerian anomalies, low birth weight (less than 7 pounds), being one of multiple fetal gestation, diethylstilbestrol (DES) exposure, endometriosis in first-degree relative, tall height, dioxin or polychlorinated biphenyls (PCB) exposure, a diet high in fat and red meat, and prior surgeries or medical therapy for endometriosis (21,22). Prior use of contraception or intrauterine device (IUD), or smoking is not associated with increased risk of endometriosis (23,24). Protective factors against the development of endometriosis include multiparity, lactation, tobacco exposure in utero, increased body mass index, increased waist-to-hip ratios, and diet high in vegetables and fruits (21,25). Some evidence suggests that women with a “pinpoint cervix” have an increased risk for endometriosis, but more studies are needed to confirm this observation (26).
Endometriosis and Cancer
Several publications link endometriosis with an increased risk for certain gynecologic and nongynecologic cancers (27,28). These associations are controversial and no good data exist to inform clinicians regarding the best management of patients who might be at risk of developing such cancers (1). Endometriosis should not be considered a medical condition associated with a clinically relevant risk of any specific cancer (29). Data from large cohort and case-control studies indicate an increased risk of ovarian cancers in women with endometriosis. The observed effect sizes are modest, varying between 1.3 and 1.9 (30). Evidence from clinical series consistently demonstrates that the association is confined to the endometrioid and clear-cell histologic types of ovarian cancer (31). A causal relationship between endometriosis and these specific histotypes of ovarian cancer should be recognized, but the low magnitude of the risk observed is consistent with the view that ectopic endometrium undergoes malignant transformation with a frequency similar to its eutopic counterpart (32). Evidence for an association with melanoma and non-Hodgkin lymphoma has been reported but needs to be verified, whereas an increased risk for other gynecologic cancer types is not supported (31).
ETIOLOGY
Although signs and symptoms of endometriosis have been described since the 1800s, its widespread occurrence was acknowledged only during the 20th century. Endometriosis is an estrogen-dependent disease. Three theories were proposed to explain the pathogenesis of endometriosis:
1. Ectopic transplantation of endometrial tissue
2. Coelomic metaplasia
The induction theory
No single theory can account for the location of endometriosis in all cases.
Transplantation Theory
The transplantation theory, originally proposed by Sampson in the mid- 1920s, is based on the assumption that endometriosis is caused by the seeding and implantation of endometrial cells by transtubal regurgitation during menstruation (33). Substantial clinical and experimental data support this hypothesis (6,34). Retrograde menstruation occurs in 70% to 90% of women, and it may be more common in women with endometriosis than in those without the disease (9,35). The presence of endometrial cells in the peritoneal fluid, indicating retrograde menstruation, is reported in 59% to 79% of women during menses or in the early follicular phase, and these cells can be cultured in vitro (36,37). The presence of endometrial cells in the dialysate of women undergoing peritoneal dialysis during menses supports the theory of retrograde menstruation (38).
Endometriosis is most often found in dependent portions of the pelvis—the ovaries, the anterior and posterior cul-de-sac, the uterosacral ligaments, the posterior uterus, and the posterior broad ligaments (39). The menstrual reflux theory combined with the clockwise peritoneal fluid current explains why endometriosis is predominantly located on the left side of the pelvis (refluxed endometrial cells implant more easily in the rectosigmoidal area) and why diaphragmatic endometriosis is found more frequently on the right side (refluxed endometrial cells implant there by the falciform ligament) (40,41).
Endometrium obtained during menses can grow when injected beneath abdominal skin or into the pelvic cavity of animals (42,43). Endometriosis was found in 50% of Rhesus monkeys after surgical transposition of the cervix to allow intra-abdominal menstruation (44). Increased retrograde menstruation by obstruction of the outflow of menstrual fluid from the uterus is associated with a higher incidence of endometriosis in women and in baboons (45–47). Women with shorter intervals between menstruation and longer duration of menses are more likely to have retrograde menstruation and are at higher risk for endometriosis (48). Menstruation is associated with intraperitoneal inflammation in women and baboons, but a limited quantity of endometrial cells can be identified in peritoneal fluid during menstruation in women, possibly because endometrial–peritoneal attachment is reported to occur within 24 hours (49–51).
Ovarian endometriosis may be caused by either retrograde menstruation or by lymphatic flow from the uterus to the ovary; metaplasia and bleeding from a corpus luteum may be a critical event in the development of some endometriomas (52–54).
Deep endometriosis, with a depth of at least 5 mm beneath the peritoneum, can present as nodules in the cul-de-sac, rectosigmoid, and bladder area and occurs with other forms of peritoneal or ovarian endometriosis (55).
According to anatomic, surgical, and pathologic findings, deep endometriotic lesions originate intraperitoneally rather than extraperitoneally. The lateral asymmetry in the occurrence of ureteral endometriosis is compatible with the menstrual reflux theory and with the anatomic differences of the left and right hemipelvis (40). Adolescents and young women can have peritoneal disease (56).
This observation, together with evidence from the development and spontaneous evolution of endometriosis in baboons, supports the notion that endometriosis starts as peritoneal disease and that the three different phenotypes and locations of endometriosis (peritoneal, ovarian, and deep) represent a homogeneous disease continuum with a single origin (i.e., regurgitated endometrium), rather than three different disease entities (40,57,58).
Extrapelvic endometriosis, although rare (1% to 2%), may result from vascular or lymphatic dissemination of endometrial cells to many gynecologic (vulva, vagina, cervix) and nongynecologic sites. The latter include bowel (appendix, colon, small intestine, hernia sacs), lungs and pleural cavity, skin (cesarean section, episiotomy or other surgical scars, inguinal region, extremities, umbilicus), lymph glands, nerves, and brain (59).
Coelomic Metaplasia
The transformation (metaplasia) of coelomic epithelium into endometrial tissue is a proposed mechanism for the origin of endometriosis. One study evaluating structural and cell surface antigen expression in the rete ovarii and epoophoron reported little commonality between endometriosis and ovarian surface epithelium, suggesting that serosal metaplasia is unlikely in the ovary (60). The results of another study involving the genetic induction of endometriosis in mice suggest that ovarian endometriotic lesions may arise directly from the ovarian surface epithelium through a metaplastic differentiation process induced by activation of an oncogenic K-ras allele (53).
Induction Theory
The induction theory is an extension of the coelomic metaplasia theory. It proposes that an endogenous (undefined) biochemical factor can induce undifferentiated peritoneal cells to develop into endometrial tissue. This theory is supported by experiments in rabbits but is not substantiated in women or nonhuman primates (61,62).
Genetic Factors
Endometriosis is a complex disorder caused by a combination of multiple genetic and environmental factors. These genetic factors need to be divided into germline and somatic genetic variants. The former is inherited and results in a higher chance of developing endometriosis, the latter are somatic alterations that possibly play a role in the pathophysiology of endometriosis.
Germline Variants
Inherited genetic variants associated with endometriosis confer a genetic susceptibility to develop the disease but represent only approximately 50% of the risk associated with the disease (63). The heritable component of endometriosis is demonstrated by familial clustering in humans and in Rhesus monkeys, a founder effect detected in the Icelandic population, higher concordance in monozygotic versus dizygotic twins, a similar age at onset of symptoms in affected nontwin sisters, an increased prevalence of endometriosis among first-degree relatives and a 15% prevalence of magnetic resonance imaging (MRI) findings suggestive of endometriosis in the first-degree relatives of women with ASRM stage III or IV disease (64). The induction of human-like endometriosis in mice by genetic activation of an oncogenic K-ras allele lends further support to the genetic basis of this disorder (53).
The risk of endometriosis is seven times greater if a first-degree relative is affected by endometriosis (65). Because no specific mendelian inheritance pattern is identified, multifactorial inheritance is postulated. Family linkage studies and genome-wide association studies (GWAS) have provided insights on the genetic variants contributing to the hereditary risk of endometriosis. Family linkage studies identify genetic variants that lead to clustering of endometriosis in certain families, but these variants are often rare in the general population. GWAS studies uncover common genetic variants in the general population related to an increased risk of endometriosis (63).
A meta-analysis of 11 GWAS identified 19 independent single nucleotide polymorphisms associated with endometriosis (66). However, all these polymorphisms combined explain only approximately 5% of variance in endometriosis. These genetic variants are located in or near a wide variety of genes with functions in diverse pathways: sex steroid hormone signaling (FSHB, ESR1), inflammation (NFE2L3), oncogenesis (ID4), uterine development (HOXA10, HOXA11), WNT signaling (WNT4, MIR148), estrogen responsive genes (GREB1, KDR) and genes involved in the actin cytoskeleton or cellular adhesion (FN1, VEZT, ANRIL). Large genome-wide linkage studies, including more than 1,300 families with multiple women affected by endometriosis, have identified three linkage regions of endometriosis: on chromosome 10q26, chromosome 20p13, and chromosome 7p13-15 (63,67,68). The genes in these linkage regions have roles in estrogen metabolism (CYP2C19 in the 10q26 region) and in endometrial or uterine development (INHBA, SFRP4 and HOXA10 in the 7p13-15 region).
Functional studies of the genes in these endometriosis risk loci are needed to elucidate their precise role and determine the effects of the variants in underlying pathways. These targeted functional gene studies have the potential to provide us with important new insights on the pathogenesis of endometriosis.
Somatic Alterations
Aneuploidy
Epithelial cells of endometriotic cysts are monoclonal on the basis of phosphoglycerate kinase gene methylation, and normal endometrial glands are monoclonal (69,70). In a comparison of endometriotic tissue with eutopic endometrium, flow cytometric DNA analysis failed to show aneuploidy (71).
Studies using comparative genomic hybridization, or multicolor in situ hybridization, showed aneuploidy for chromosomes 11, 16, and 17, increased heterogeneity of chromosome 17 aneuploidy, and losses of 1p and 22q (50%), 5p (33%), 6q (27%), 70 (22%), 9q (22%), and 16 (22%) of 18 selected endometriotic tissues (72–74). In another study, trisomies 1 and 7, and monosomies 9 and 17 were found in endometriosis, ovarian endometrioid adenocarcinoma, and normal endometrium (75). The proportions of aneusomic cells were significantly higher in ovarian endometriosis compared with extragonadal endometriosis and normal endometrium (p <0.001), suggesting a role of the ovarian stromal milieu in the induction of genetic changes, which may lead to invasive cancer in isolated cases (75).
Microsatellite DNA assays reveal an allelic imbalance (loss of heterozygosity) in p16 (Ink4), GALT, p53, and APOA2 loci in patients with endometriosis and in stage II of endometriosis (76). Another report found a loss of heterozygosity in 28% of endometriotic lesions at one or more sites: chromosomes 9p (18%), 11q (18%), and 22q (15%) (70).
Somatic Mutations
Somatic mutations in genes or chromosomes are, by definition, never passed on to progeny but can play an important role in pathogenesis of diseases such as cancer.
The role of somatic mutations in endometriosis is not well understood. Patients with clear cell ovarian carcinoma or endometrioid ovarian carcinoma often have concomitant endometriosis and endometriosis is well recognized as a risk factor for these types of ovarian cancer. Investigation of endometriosis lesions occurring synchronously with clear cell ovarian carcinoma or endometrioid ovarian carcinoma showed somatic mutations in ARID1A, PIK3CA, and in the MET oncogenes (77–79). However, data on the presence of somatic alterations in noncancer-associated endometriosis are very limited. One study revealed the presence of somatic mutations in noncancer-associated deep endometriotic lesions in 19 of 24 patients (79%) (80). Five patients harbored known cancer driver mutations in ARID1A, PIK3CA, KRAS, or PPP2R1A although deep infiltrating endometriosis has virtually no risk for malignant transformation. All the tested somatic mutations appeared to be confined to the epithelial compartment of endometriotic lesions.
Immunologic Factors and Inflammation
Retrograde menstruation appears to be a common event in women and not all women who have retrograde menstruation develop endometriosis. The immune system may be altered in women with endometriosis, and it is hypothesized that the disease may develop as a result of reduced immunologic clearance of viable endometrial cells from the pelvic cavity (81,82). Endometriosis can be caused by decreased clearance of peritoneal fluid endometrial cells resulting from reduced natural killer (NK) cell activity or decreased macrophage activity (83). Decreased cell-mediated cytotoxicity toward autologous endometrial cells is associated with endometriosis (83–87). These studies used techniques that have considerable variability in target cells and methods (88,89). Whether NK cell activity is lower in patients with endometriosis than in those without endometriosis is controversial. Some reports demonstrate reduced NK activity and others found no increase in NK activity in women with moderate to severe disease (85–87,90–95). There is great variability in NK cell activity among normal individuals that may be related to variables such as smoking, drug use, and exercise (88).
In contrast, endometriosis can be considered a condition of immunologic tolerance, as opposed to ectopic endometrium, which essentially is self-tissue (81). It can be questioned why viable endometrial cells in the peritoneal fluid would be a target for NK cells or macrophages. Autotransplantation of blood vessels, muscles, skin grafts, and other tissues is extremely successful (84–86).
There is no in vitro evidence that peritoneal fluid macrophages actually attack and perform phagocytosis of viable peritoneal fluid endometrial cells. High-dose immunosuppression can slightly increase the progression of spontaneous endometriosis in baboons (96). There is no clinical evidence that the prevalence of endometriosis is increased in immunosuppressed patients. The fact that women with kidney transplants, who undergo chronic immunosuppression and are not known to have increased infertility problems can be considered indirect evidence that these patients do not develop extensive endometriosis.
Substantial evidence suggests that endometriosis is associated with a state of subclinical peritoneal inflammation, marked by an increased peritoneal fluid volume, increased peritoneal fluid white blood cell concentration (especially macrophages with increased activation status), and increased inflammatory cytokines, growth factors, and angiogenesis-promoting substances. It is reported in baboons that subclinical peritoneal inflammation occurs during menstruation and after intrapelvic injection of endometrium (94). A higher basal activation status of peritoneal macrophages in women with endometriosis may impair fertility by reducing sperm motility, increasing sperm phagocytosis, or interfering with fertilization, possibly by increased secretion of cytokines such as tumor necrosis factor α (TNF-α) (97–101). Tumor necrosis factor may facilitate the pelvic implantation of ectopic endometrium (100,101).
The adherence of human endometrial stromal cells to mesothelial cells in vitro is increased by the pretreatment of mesothelial cells with physiologic doses of TNF- α (102). Macrophages or other cells may promote the growth of endometrial cells by secretion of growth and angiogenetic factors such as epidermal growth factor (EGF), macrophage-derived growth factor (MDGF), fibronectin, and adhesion molecules such as integrins (102–108). After attachment of endometrial cells to the peritoneum, subsequent invasion and growth appear to be regulated by matrix metalloproteinases (MMP) and their tissue inhibitors (109,110).
There is increasing evidence that local inflammation and secretion of prostaglandins (PGs) is related to differences in endometrial aromatase activity between women with and without endometriosis. Expression of aromatase cytochrome P450 protein and mRNA is present in human endometriotic implants but not in normal endometrium, suggesting that ectopic endometrium produces estrogens, which may be involved in the tissue growth interacting with the estrogen receptor (111). Inactivation of 17β-estradiol is impaired in endometriotic tissues because of deficient expression of 17β-hydroxysteroid dehydrogenase type 2, which is normally expressed in eutopic endometrium in response to progesterone (112). The inappropriate aromatase expression in endometriosis lesions can be stimulated by prostaglandin E2 (PGE2). This reaction leads to local production of E2, which stimulates PGE2 production, resulting in a positivefeedback system between local inflammation and estrogen-driven local growth of ectopic endometrium (113).
The subclinical pelvic inflammatory status associated with endometriosis is reflected in the systemic circulation. Increased concentrations of C-reactive protein, serum amyloid A (SAA), TNF-α, membrane cofactor protein-1, interleukin-6 (IL-6), IL-8, and chemokine (C-C motif) receptor 1 (CCR1) are observed in peripheral blood samples of patients with endometriosis when compared with controls (114). This observation offers a basis for the development of noninvasive diagnostic tests.
Both hypothesis-driven research and system biology approaches using mRNA microarray and proteomic techniques studies show that eutopic endometrium is biologically different in women with endometriosis when compared to controls with respect to proliferation, apoptosis, angiogenesis, and inflammatory pathways (115–118). Several studies show a higher prevalence of nerve fibers and neurotrophic factors in the eutopic endometrium from women with endometriosis when compared to controls (49,119).
Environmental Factors and Dioxin
There is an increasing awareness of potential links between reproductive health, infertility, and environmental pollution. Attention was directed toward the potential role of dioxins in the pathogenesis of endometriosis, but the issue remains controversial. A meta-analysis concluded that there is insufficient evidence in women or in nonhuman primates that endometriosis is caused by dioxin exposure (120).
Human Data
A 1976 explosion of a factory in Seveso, Italy, resulted in the highest recorded levels of dioxin exposure in humans, but data are not published (121). The Seveso Women’s Health Study will correlate prospective individual data on exposure to dioxin with reproductive endpoints such as the incidence of endometriosis, infertility, and decreased sperm quality. One case-control study failed to show an association in the general population between endometriosis and exposure to PCB and chlorinated pesticides during adulthood. No differences in mean plasma concentrations of 14-PCB and 11-chlorinated pesticides were found between women with and those without endometriosis (122). In another study, increased exposure to dioxin-like compounds is associated with (moderate to severe) endometriosis in a case-control study in women (123). Genetic mechanisms may play a role in dioxin exposure and the development of endometriosis. Transcripts of the CYP1A1 gene, a dioxin-induced gene, are significantly higher (nine times higher) in endometriotic tissues than in eutopic endometrium (113). Other investigators report a similar expression of aryl hydrocarbon receptor and dioxinrelated genes (using semiquantitative reverse transcriptase polymerase chain reaction) in the endometrium from women with or without endometriosis (124).
In Japanese women, no association was found between endometriosis prevalence or severity and polymorphisms for aryl hydrocarbon receptor repressor, aryl hydrocarbon (x2) receptor, and aryl hydrocarbon nuclear translocator or CYP1A1 genes (125). Based on these data, there is insufficient evidence supporting the association between endometriosis and dioxin exposure in humans.
Nonhuman Primates
An initial retrospective case-control study reported that the prevalence of endometriosis was not statistically different (p = 0.08) between monkeys chronically exposed to dioxin for 4 years (11 of 14, 79%) and unexposed animals (2 of 6, 33%) after a period of 10 years. A positive correlation was found between the severity of endometriosis and dioxin dose, serum levels of dioxin, and dioxinlike chemicals (126,127). Two prospective studies evaluated the association between dioxin exposure and development of endometriosis in Rhesus monkeys.
In one study, monkeys exposed for 12 months to low-dose dioxin (0.71 ng/kg/day) had endometriosis implants with smaller maximal and minimal diameters and similar survival rate when compared with endometriotic lesions in unexposed controls, suggesting no effect of dioxin on endometriosis (128). After 12 months of exposure to high-dose dioxin (17.86 ng/kg/day), larger diameters and a higher survival rate of endometriosis implants were observed in exposed
Rhesus monkeys compared with unexposed controls. The second randomized study performed in 80 Rhesus monkeys compared those with no treatment with those treated with 0, 5, 20, 40, and 80 μg of aroclor (1,254 kg/day) for 6 years. Endometriosis occurred in 37% of controls and in 25% of treated monkeys as determined by laparoscopy and necropsy data (129). No association was observed between endometriosis severity and PCB exposure. These data question the importance of dioxin exposure, except at high doses, in the development of endometriosis in primates.
Rodents
Continuous exposure to 2,3,7,8-tetrachlorodibenzo-P-dioxin inhibited the growth of surgically induced endometriosis in ovariectomized mice treated with highdose estradiol. No correlation was observed between the dose of dioxin and survival of endometrial implants, adhesions, and serum E2 levels (130). In ovariectomized mice induced with endometriosis, similar stimulating effects of estrone and 4-chlorodiphenyl ether (4-CDE) were observed on survival rates of endometriotic mice, suggesting an estrogen-like effect of 4-CDE (131). Potential mechanisms mediating dioxin action to promote endometriosis in rodents are complex and probably different in rats and mice, and furthermore in women. The mouse appears to be a better model to elucidate these mechanisms, but both models have important limitations (132,133).
Stem Cells
In 2004 the first evidence for the presence of adult stem cells in endometrium was published (134). In one study, stem cells were identified in endometrium isolated from hysterectomy tissue. Concurrently, it was reported that some epithelial and stromal cells in human endometrium of bone marrow transplant recipients were of donor origin (135). In the next decade, a key role of adult stem cells in normal endometrial physiology was discovered (136).
Following the discovery of endometrial stem cells, it was proposed that these stem cells play a role in the pathogenesis of endometriosis. The first hypothesis is an extension of Sampson theory of retrograde menstruation: endometrial stem cells, with intrinsic abnormalities such as germline or somatic mutations, may have increased propensity to implant on the peritoneum after retrograde menstruation. Studies showed that women with endometriosis shed basalis endometrium at menstruation, increasing the likelihood of endometrial epithelial progenitor cells gaining access to the peritoneal cavity and stem cells have been identified in endometriotic lesions (136). However, the presence of endometrial stem/progenitor cells in peritoneal fluid has not yet been reported, which is crucial for identifying their role in the pathogenesis of endometriosis. A second hypothesis focuses on abnormal stem cell recruitment in patients with endometriosis (137). Stem cells from the bone marrow or alternate sources could be preferentially recruited in ectopic over eutopic endometrium through altered expression of chemotactic ligands such as CXCL12 (137). Abnormal endometrial function caused by impaired recruitment of stem cells to the eutopic endometrium could be a contributing endometrial factor to endometriosis-associated infertility (137).
Several studies have addressed the role of stem cells in endometriosis, but no strong direct evidence for the role of endometrial stem/progenitor cells in the pathogenesis of endometriosis has been reported.
Future Research
The study of endometriosis is compounded by the need to determine the presence or absence of pathology. The pathogenesis of endometriosis, the pathophysiology of related infertility, and the spontaneous evolution of endometriosis are being studied. At the time of diagnosis, most patients with endometriosis had the disease for an unknown period, making it difficult to initiate clinical experiments that would determine the etiology or progression of the disease (34). Because endometriosis occurs naturally only in women and nonhuman primates, and invasive experiments cannot be performed easily, it is difficult to undertake properly controlled studies.
There is a need for the development of good in vitro and in vivo models for endometriosis. The main advantage of the rodent models used to study endometriosis is their low cost relative to nonhuman primates, but the disadvantages are numerous (138–141). First of all, in these models, the type of lesion appears to be quite different from the variety of pigmented and nonpigmented lesions observed in women (138–140). Secondly, there is a lack of standardization in rodent endometriosis models and no consensus on relevant outcome measures. Nonhuman primates are phylogenetically close to humans, have a comparable menstrual cycle, are afflicted with spontaneous endometriosis, and when induced with endometriosis, develop macroscopic lesions that are similar to those found in human disease (44,142–146). Spontaneous endometriosis in the baboon is minimal and disseminated, similar to the different stages of endometriosis in women (142,147–149).
The techniques for in vitro experiments have significantly improved, including the development of endometrial organoids (150,151). Application of these promising new techniques on endometriotic tissue and further improvement of in vitro models for endometriosis hold great potential for future research.
DIAGNOSIS
Clinical Presentation
[2] Endometriosis should be suspected in women with infertility, dysmenorrhea, dyspareunia, or chronic pelvic pain, although these symptoms can be associated with other diseases. Endometriosis may be asymptomatic, even in women with more advanced disease (i.e., ovarian endometriosis or deep endometriosis).
Endometriosis can be associated with significant gastrointestinal symptoms (pain, nausea, vomiting, early satiety, bloating and distention, altered bowel habits). A characteristic motility change (ampulla of Vater– duodenal spasm, a seizure equivalent of the enteric nervous system, along with bacterial overgrowth) is documented in most women with the disease (152).
Women of reproductive age with endometriosis are not osteopenic (153). The average delay between onset of pain symptoms and surgically confirmed endometriosis is quite long: 8 years or longer in the United
Kingdom and 9 to 12 years in the United States (154). Similar durations were observed in Scandinavia and in Brazil (155,156). A delay in diagnosis of endometriosis of 6 and 3 years in women with pain and women with infertility, respectively, was reported. Over previous decades, there was a steady decrease in the delay in diagnosis and a decline in the prevalence of advanced endometriosis at first diagnosis (157). Patient awareness of endometriosis was increased. Many patients’ quality of life is affected by pain, emotional impact of infertility, anger about disease recurrence, and uncertainty about the future regarding repeated surgeries or long-term medical therapy and its side effects (158). Endometriosis should be perceived as a chronic disease, at least in a subset of highly symptomatic women, and quality-of-life should be evaluated using reliable and validated questionnaires (159).
Pain
[3] In adult women, dysmenorrhea may be especially suggestive of endometriosis if it begins after years of pain-free menses. Dysmenorrhea often starts before the onset of menstrual bleeding and continues throughout the menstrual period. In adolescents, the pain may be present after menarche without an interval of pain-free menses. Evidence suggests that absenteeism from school and the incidence and duration of oral contraceptive (OC) use for severe primary dysmenorrhea during adolescence is higher in women who later develop deep endometriosis than in women without deep endometriosis (160).
The distribution of pain is variable but most often is bilateral. Local symptoms can arise from rectal, ureteral, and bladder involvement, and lower back pain can occur. Some women with extensive disease have no pain, whereas others with only minimal to mild disease may experience severe pelvic pain. All endometriosis lesion types are associated with pelvic pain, including minimal to mild endometriosis (161). Endometriomas are not associated with dysmenorrheal severity, and dysmenorrhea is less frequent in women with only ovarian endometriomas compared with other locations (162,163). Endometriomas can be considered a marker for greater severity of deep lesions (164). Deep lesions are consistently associated with pelvic pain, gastrointestinal symptoms, and painful defecation (165). The role of adhesions in pain and endometriosis is poorly understood (166).
Many studies failed to detect a correlation between the degree of pelvic pain and the severity of endometriosis (12,163,167). Some studies reported a positive correlation between endometriosis stage and endometriosis-related dysmenorrhea or chronic pelvic pain (168,169). In one study, a significant but weak correlation was observed between endometriosis stage and severity of dysmenorrhea and nonmenstrual pain, whereas a strong association was found between posterior cul-de-sac lesions and dyspareunia (170).
Possible mechanisms causing pain in patients with endometriosis include local peritoneal inflammation, deep infiltration with tissue damage, adhesion formation, fibrotic thickening, and collection of shed menstrual blood in endometriotic implants, resulting in painful traction with the physiologic movement of tissues (171,172). The character of pelvic pain is related to the anatomic location of deep endometriotic lesions (165). Severe pelvic pain and dyspareunia may be associated with deep endometriosis (7,171,173). In rectovaginal endometriotic nodules, a close histologic relationship was observed between nerves and endometriotic foci and between nerves and the fibrotic component of the nodule (174). Increasing evidence suggests a close relationship between the density of innervation of endometriotic lesions and pain symptoms (170).
Infertility
Many arguments support the hypothesis that there is a causal relationship between the presence of endometriosis and infertility (175). The following factors have been reported:
1. Increased prevalence of endometriosis in infertile women (33%) when compared to women of proven fertility (4%), a reduced monthly fecundity rate (MFR) in baboons with mild to severe (spontaneous or induced) endometriosis when compared to those with minimal endometriosis or a normal pelvis.
2. Trend toward a reduced MFR in infertile women with minimal to mild endometriosis when compared to women with unexplained infertility.
3. Endometriotic ovarian cysts that negatively affect the rate of spontaneous ovulation (176).
4. Dose–effect relationship: A negative correlation between the r-AFS stage of endometriosis and the MFR and cumulative pregnancy rate (175,177).
5. Reduced MFR and cumulative pregnancy rate after donor sperm insemination in women with minimal to mild endometriosis when compared to those with a normal pelvis.
6. Reduced MFR after husband sperm insemination in women with minimal to mild endometriosis when compared to those with a normal pelvis.
7. Reduced implantation rate per embryo after in vitro fertilization (IVF) in women with endometriosis when compared to women with tubal factor infertility (175,178).
8. Increased MFR and cumulative pregnancy rate after surgical removal of minimal to mild endometriosis.
When endometriosis is moderate or severe, involving the ovaries and causing adhesions that block tubo-ovarian motility and ovum pickup, it is associated with infertility (176,179). This effect was shown in primates, including cynomolgus monkeys and baboons (145,180). Numerous mechanisms (ovulatory dysfunction, luteal insufficiency, luteinized unruptured follicle syndrome, recurrent abortion, altered immunity, and intraperitoneal inflammation) are proposed as explanations, but an association between fertility and minimal or mild endometriosis remains controversial (181).
Spontaneous Abortion
A possible association between endometriosis and spontaneous abortion was suggested in uncontrolled or retrospective studies. Some controlled studies evaluating the association between endometriosis and spontaneous abortion have important methodologic shortcomings: heterogeneity between cases and controls, analysis of the abortion rate before the diagnosis of endometriosis, and selection bias of study and control groups (81,182,183). Based on controlled prospective studies, there is no evidence that endometriosis is associated with (recurrent) pregnancy loss or that medical or surgical treatment of endometriosis reduces the spontaneous abortion rate (184–186). Some data suggest that miscarriage rates may be increased after treatment with assisted reproductive technology (187).
Endocrinologic Abnormalities
Endometriosis is associated with anovulation, abnormal follicular development with impaired follicle growth, reduced circulating E2 levels during the preovulatory phase, disturbed luteinizing hormone (LH) surge patterns, premenstrual spotting, luteinized unruptured follicle syndrome, and galactorrhea, and hyperprolactinemia (188). Increased incidence and recurrence of the luteinized unruptured follicle syndrome is reported in baboons with mild endometriosis, but not in primates with minimal endometriosis or a normal pelvis (189). Luteal insufficiency with reduced circulating E2 and progesterone levels, out-of-phase endometrial biopsies, and aberrant integrin expression was reported in the endometrium of women with endometriosis by some researchers, but these findings were not confirmed by other investigators (188,190,191). No convincing data exist to conclude that the incidence of these endocrine abnormalities is increased in women who have endometriosis.
Extrapelvic Endometriosis
Extrapelvic endometriosis, although often asymptomatic, should be suspected when symptoms of pain or a palpable mass occur outside the pelvis in a cyclic pattern. Endometriosis involving the intestinal tract (especially colon) is the most common site of extrapelvic disease and may cause abdominal and back pain, abdominal distention, cyclic rectal bleeding, constipation, and obstruction. Ureteral involvement can lead to obstruction and result in cyclic pain, dysuria, and hematuria. Endometriosis lesions on the diaphragm often result in cyclic shoulder pain. Pulmonary endometriosis can manifest as pneumothorax, hemothorax, or hemoptysis during menses. Umbilical endometriosis should be suspected when a patient has a palpable mass and cyclic pain in the umbilical area (59).
Clinical Examination
In many women with endometriosis, no abnormality is detected during the clinical examination. However, the vulva, vagina, and cervix should be inspected for any signs of endometriosis, although the occurrence of endometriosis in these areas is rare (e.g., episiotomy scar). The presence of a narrow pinpoint cervical ostium can be a risk factor for endometriosis (26). Other signs of possible endometriosis include uterosacral or cul-de-sac nodularity, lateral or cervical displacement caused by uterosacral scarring, painful swelling of the rectovaginal septum, and unilateral ovarian cystic enlargement (192). In more advanced disease, the uterus is often in fixed retroversion, and the mobility of the ovaries and fallopian tubes is reduced (“frozen pelvis”). Evidence of deep endometriosis (deeper than 5 mm under the peritoneum) in the rectovaginal septum with cul-de-sac obliteration or cystic ovarian endometriosis should be suspected when there is clinical documentation of uterosacral nodularities during menses (193–195). In these cases, black-blue– colored lesions can sometimes be observed in the vagina during speculum examination.
Imaging
Based on the symptoms at presentation and the clinical examination the diagnosis of endometriosis can be suspected. However, endometriosis symptoms are not specific and the clinical examination may have false-negative results. The next step in the diagnostic workup is medical imaging. However, no imaging modality detects endometriosis accurately enough to replace surgical visual detection and biopsy for diagnosis and thus the gold standard for diagnosis of endometriosis remains laparoscopic visualization of lesions with histologic confirmation (196).
Ultrasound
The guidelines from the European Society of Human reproduction and Embryology (ESHRE) and American College of Obstetricians and Gynecology (ACOG) recommend transvaginal ultrasound (TVUS) as a first imaging step in the diagnostic work up of women with suspected endometriosis. However, the sensitivity and specificity of TVUS for diagnosis of endometriosis are strongly dependent on the interest and experience of the sonographer and on the quality of the ultrasound equipment.
In a systematic review the accuracy of TVUS for detecting the different phenotypes of endometriosis (peritoneal, endometrioma, deep infiltrating) was assessed (196). TVUS could not reliably visualize peritoneal endometriosis (sensitivity = 65%, specificity = 95%). Compared to laparoscopy, TVUS has no value in diagnosing peritoneal endometriosis. However, TVUS is reliable in detecting or excluding the presence of an endometrioma (sensitivity = 93%, specificity = 96%). The typical ultrasound features of an endometriotic ovarian cyst in premenopausal women were described as “ground-glass echogenicity of the cyst fluid, one to four locules and no solid parts” (197). Studies evaluating the accuracy of TVUS for diagnosis of deep endometriosis mainly focus on rectovaginal or bladder nodules. TVUS can detect these deep endometriotic lesions with moderate reliability (sensitivity = 79%, specificity = 94%). The role of TVUS in diagnosis of deep endometriosis in more distant locations is limited.
In summary, the main role of TVUS in the diagnostic workup of endometriosis is detecting the presence of an endometrioma or deep endometriotic nodule
because it establishes the diagnosis with high certainty. Moreover, an
endometrioma or a deep nodule are very rarely isolated findings. Patients
with an endometrioma or a deep nodule often have other endometriotic lesions
and adhesions. Identification of an endometrioma or a deep nodule on TVUS
should always be followed by a detailed investigation for other (peritoneal and
deep) endometriotic lesions.
Local guidelines for the management of suspected ovarian malignancy should be followed in cases of ovarian endometrioma (1). Ultrasound scanning with or
without serum CA125 testing is usually used to identify rare instances of ovarian
cancer; however, CA125 levels are frequently elevated in the presence of
endometriomas (1).
The absence of endometriosis on TVUS does not rule out the presence of peritoneal or deep endometriosis. Based on the patients’ symptoms and
clinical examination further investigation and a diagnostic laparoscopy
should be considered.
Other Imaging Techniques
Other imaging techniques, including computed tomography (CT) and MRI, can be used to provide additional and confirmatory information, but they are not considered first line imaging modalities because of the high costs and their added value is unclear (1). Moreover, CT results in an important radiation dose, which should be avoided as much as possible in a population of young reproductive women.
MRI has a good sensitivity and specificity for the diagnosis of deep endometriosis and endometriomas, but the value in addition to TVUS is limited. A growing number of studies suggest that MRI has a role in the diagnosis of endometriosis because of a greater ability to detect small lesions and lesions on distant locations (extrapelvic endometriosis) (196). However, a negative MRI does not rule out peritoneal endometriosis because lesions are identified only if they are hemorrhagic, greater than 5 mm or when associated with extensive adhesions distorting the normal anatomy. Hysterosalpingography is not recommended as a diagnostic test for endometriosis, although the presence of filling defects (presence of hypertrophic or polypoid endometrium) has a significant positive correlation with endometriosis (positive and negative predictive values of 84% and 75%, respectively) (198).
Assessment of Intestinal and Urologic Involvement
Medical imaging plays an important role in establishing the diagnosis of endometriosis and imaging-based mapping of the extent of endometriosis is indispensable for appropriate planning of surgical management. If there is clinical evidence of deep endometriosis, ureteral, bladder, and bowel involvement should be assessed. Ureteral involvement may be asymptomatic in up to 50% of patients with deep endometriosis (199). Consideration should be given to performing ultrasound (transrectal, transvaginal, or renal), a CT urogram, or an MRI. A barium enema study might be useful, depending on the individual circumstances, to map the extent of disease present, which may be multifocal (1). There is no proof that one technique is superior to another; it is recommended that the technique that is most familiar to the radiologist involved be used.
Blood and Other Tests
There is no specific blood test for the diagnosis of endometriosis. A general endometriosis screening test may be neither appropriate (risk for overdiagnosis)
nor feasible. A blood test with a high sensitivity would be useful if that would
identify women with symptomatic endometriosis (pelvic pain, infertility) that is
not detectable by ultrasound imaging (200). This would include all cases of
minimal to mild endometriosis and those cases of moderate to severe
endometriosis without detectable ovarian endometriotic cysts or nodules (201).
These are patients who could benefit from laparoscopic surgery to reduce
endometriosis-associated pain and infertility or to diagnose and treat other pelvic
causes of pelvic pain or infertility, like pelvic adhesions. From that perspective, a
lower specificity would be acceptable because the main goal of such a test would
be to rule in all women with potential endometriosis or other pelvic diseases who
might benefit from surgery (202).
CA125
Levels of CA125, a glycoprotein from coelomic epithelium and common to most nonmucinous epithelial ovarian carcinomas, are significantly higher in
women with moderate or severe endometriosis and normal in women with
minimal or mild disease (203,204). It is presumed that endometriosis lesions
produce peritoneal irritation and inflammation and this leads to an increased
shedding of CA125 (204). During menstruation, an increase in CA125 levels was
shown in women with and without endometriosis (205–209). Other studies did
not find an increase during menses, or found an increase only with moderate to
severe endometriosis (210–213). The levels of CA125 vary widely: in patients
without endometriosis (8 to 22 U/mL in the nonmenstrual phase), in those with
minimal to mild endometriosis (14 to 31 U/mL in the nonmenstrual phase), and in
those with moderate to severe disease (13 to 95 U/mL in the nonmenstrual phase).
Compared with laparoscopy, measurement of serum CA125 levels has no
value as a diagnostic tool (214).
Laparoscopy
General Considerations
Unless disease is visible in the vagina or elsewhere, laparoscopy is the standard technique for visual inspection of the pelvis and establishment of a
definitive diagnosis (1). There is insufficient evidence to justify timing the
laparoscopy at a specific time in the menstrual cycle. Laparoscopic recognition of
endometriosis will vary with the experience of the surgeon, especially for subtle
bowel, bladder, ureteral, and diaphragmatic lesions (1). A meta-analysis of its
value against a histologic diagnosis showed (assuming a 10% pretest probability
of endometriosis) that a positive laparoscopy increases the likelihood of disease to
32% (95% confidence interval [CI], 21–46) and a negative laparoscopy decreases
the likelihood to 0.7% (95% CI, 0.1–5.0) (1,215). Diagnostic laparoscopy is
associated with an approximately 3% risk of minor complications (e.g., nausea,
shoulder tip pain) and a risk of major complications (e.g., bowel perforation,
vascular damage) of 0.6 to 1.8 per 1,000 cases (1,216,217). Endometriosis can be
treated during laparoscopy, thus combining diagnosis and therapy.
Laparoscopic Technique
During diagnostic laparoscopy, the pelvic and abdominal cavity should be systematically investigated for the presence of endometriosis. This
examination should include a complete inspection and palpation with a blunt
probe to check for nodularity as a sign of deep endometriosis of the bowel,
bladder, uterus, tubes, ovaries, cul-de-sac, or broad ligament (Fig. 13-1). The
type, location, and extent of all lesions and adhesions should be documented in
the operative notes; ideally, the findings should be recorded with photographs or on video (1).
Laparoscopic Findings
The laparoscopic findings of endometriosis include peritoneal lesions, ovarian
endometriotic cysts, and deep endometriosis invading the peritoneal surface with
a depth of at least 5 mm. Most patients with ovarian endometriotic cysts or
deep endometriosis also have peritoneal disease.
Peritoneal Endometriosis
Characteristic findings include typical (“powder-burn” or “gunshot”) lesions
on the serosal surfaces of the peritoneum. These lesions are black, dark
brown, or bluish nodules or small cysts containing old hemorrhage
surrounded by a variable degree of fibrosis (Fig. 13-2). Endometriosis can
appear as subtle lesions, including red implants (petechial, vesicular, polypoid,
hemorrhagic, red flame-like), serous or clear vesicles, white plaques or scarring,
yellow-brown discoloration of the peritoneum, and subovarian adhesions (Fig.
13-3) (139,140,142,218,219). Histologic confirmation of the laparoscopic
impression is essential for the diagnosis of endometriosis, for subtle lesions, and
for the typical lesions reported to be histologically negative in 24% of cases
(220,221).
Deep Endometriosis
Mild forms of deep endometriosis may be detected only by palpation under an
endometriotic lesion or by discovery of a palpable mass beneath visually normal
peritoneum, most notably in the posterior cul-de-sac (Fig. 13-4) (194). At
laparoscopy, deep endometriosis may have the appearance of minimal
disease, resulting in an underestimation of disease severity (194). Reduced
size of the cul-de-sac in women with deep endometriosis suggests that such
lesions develop not in the rectovaginal septum but intraperitoneally and that burial
of anterior rectal wall adhesions creates a false bottom, giving an erroneous
impression of extraperitoneal origin (222).
FIGURE 13-1 Pelvic endometriosis.
Ovarian Endometriosis
The diagnosis of ovarian endometriosis is facilitated by careful inspection of
all sides of both ovaries, which may be difficult when adhesions are present
in more advanced stages of disease (Fig. 13-5). With superficial ovarian
endometriosis, lesions can be both typical and subtle. Larger ovarian
endometriotic cysts (i.e., endometriomas) usually are located on the anterior
surface of the ovary and are associated with retraction, pigmentation, and
adhesions to the posterior peritoneum. These ovarian endometriotic cysts often
contain a thick, viscous dark brown fluid (i.e., “chocolate fluid”), composed
of hemosiderin derived from previous intraovarian hemorrhage. Because this
fluid may be found in other conditions, such as in hemorrhagic corpus luteum
cysts or neoplastic cysts, biopsy and preferably removal of the ovarian cyst for
histologic confirmation are necessary for the diagnosis in the revised
endometriosis classification of the American Society for Reproductive Medicine (ASRM). If that is not possible, the presence of an ovarian endometriotic cyst
should be confirmed by the following features: cyst diameter of less than 12 cm,
adhesion to pelvic sidewall or broad ligament, endometriosis on the surface of the
ovary, and tarry, thick, chocolate-colored fluid content (223). Ovarian
endometriosis appears to be a marker for more extensive pelvic and intestinal
disease. Exclusive ovarian disease is found in only 1% of endometriosis patients,
with the remaining patients having extensive pelvic or intestinal endometriosis
(224).
Histologic Confirmation
[1] Positive histology confirms the diagnosis of endometriosis; negative histology does not exclude it (1). Whether histology should be obtained when peritoneal disease alone is present is controversial; visual inspection is usually adequate but histologic confirmation of at least one lesion is ideal (1).
In cases of ovarian endometrioma (>4 cm in diameter) and in deep endometriosis, histology is recommended to exclude rare instances of malignancy (1).
FIGURE 13-2 Typical and subtle endometriotic lesions on peritoneum. A: Typical blackpuckered lesions with hypervascularization and orange polypoid vesicles. B: Red polypoid lesions with hypervascularization. (Photographs from Dr. Christel Meuleman, Leuven University Fertility Center, Leuven University Hospitals, Leuven, Belgium.)
FIGURE 13-3 Ovarian endometriosis. A: Superficial ovarian endometriosis. B: Superficial ovarian endometriosis and endometrioma—laparoscopic image prior to adhesiolysis. C: Laparoscopic image of uterus and right ovary with dark endometrioma. D:
Ovarian endometriotic cystectomy. E: Ovarian endometriotic cystectomy. (Photographs from Dr. Christel Meuleman, Leuven University Fertility Center, Leuven University Hospitals, Leuven, Belgium.)
FIGURE 13-4 Laparoscopic excision of deep endometriosis from the cul-de-sac. A: Extensive endometriosis with deep nodule at the right uterosacral ligament, masked by adhesions. B: Deep nodule still present in dense adhesion between rectum and uterosacral ligaments. C: Cul-de-sac after resection of deep nodule with CO2 laser. (Photographs from Dr. Christel Meuleman, Leuven University Fertility Center, Leuven University Hospitals, Leuven, Belgium.)
FIGURE 13-5 Revised American Society for Reproductive Medicine Classification. (From the American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis. Am Soc Reprod Med 1997;5:817– 821.)
In a study of 44 patients with chronic pelvic pain, endometriosis was laparoscopically diagnosed in 36%, but histologic confirmation was obtained in
only 18%. This approach resulted in a low diagnostic accuracy of laparoscopic
inspection with a positive predictive value of only 45%, explained by a specificity
of only 77% (225).
Microscopically, endometriotic implants consist of endometrial glands
and/or stroma, with or without hemosiderin-laden macrophages (Fig. 13-6).
It is suggested that using these stringent and unvalidated histologic criteria may
result in significant underdiagnosis of endometriosis (6). Problems in obtaining
biopsies (especially small vesicles) and variability in tissue processing (step or
partial instead of serial sectioning) may contribute to false-negative results.
Endometrioid stroma may be more characteristic of endometriosis than
endometrioid glands (226). The presence of stromal endometriosis, which
contains endometrial stroma with hemosiderin-laden macrophages or hemorrhage,
was reported in women and in baboons and may represent a very early event in
the pathogenesis of endometriosis (148,220,221). Isolated endometrial stromal
cell nodules, immunohistochemically positive for vimentin and estrogen receptor,
can be found in the absence of endometrial glands along blood or lymphatic
vessels (227).
FIGURE 13-6 Histologic appearance of endometriosis: endometrial glandular epithelium, surrounded by stroma in typical lesion and clear vesicle.
Different types of lesions may have different degrees of proliferative or secretory glandular activity (226). Vascularization, mitotic activity, and the threedimensional structure of endometriosis lesions are key factors (171,228,229). Deep endometriosis is described as a specific type of pelvic endometriosis characterized by proliferative strands of glands and stroma in dense fibrous and smooth muscle tissue (20). Smooth muscles are frequent components of endometriotic lesions on the peritoneum, ovary, rectovaginal septum, and uterosacral ligaments (174).
Microscopic endometriosis is defined as the presence of endometrial glands and stroma in macroscopically normal pelvic peritoneum. It is important in the histogenesis of endometriosis and its recurrence after treatment (230,231). The clinical relevance of microscopic endometriosis is controversial
because it is not observed uniformly. Using undefined criteria for what constitutes
normal peritoneum, peritoneal biopsy specimens of 1 to 3 cm were obtained
during laparotomy from 20 patients with moderate to severe endometriosis (231).
Examination of the biopsy results with low-power scanning electron microscopy
revealed unsuspected microscopic endometriosis in 25% of cases not confirmed
by light microscopy. Peritoneal endometriotic foci were demonstrated by light
microscopy in areas that showed no obvious evidence of disease (232).
In serial sections of laparoscopic biopsies of normal peritoneum, 10% to
15% of women had microscopic endometriosis, and endometriosis was found
in 6% of those without macroscopic disease (219,233,234). Other studies were
unable to detect microscopic endometriosis in 2-mm biopsy specimens of visually
normal peritoneum (235–238). Examination of larger samples (5 to 15 mm) of
visually normal peritoneum revealed microscopic endometriosis in only 1 of 55
patients studied (239). A histologic study of serial sections through the entire
pelvic peritoneum of visually normal peritoneum from baboons with and without
disease indicated that microscopic endometriosis is a rare occurrence (96).
Macroscopically appearing normal peritoneum rarely contains microscopic
endometriosis (239).
Laparoscopic Classification
Endometriosis is a complex disease and at present there is no perfect staging system available. The most widely used staging system is the revised
American Society for Reproductive Medicine classification (rASRM). The
Endometriosis Fertility Index (EFI) has been shown to predict non-IVF pregnancy
rates for patients following surgical staging and treatment of endometriosis
(177,240). To supplement the rASRM classification with regard to the description
of deep endometriosis, the ENZIAN score was introduced (241–243). Although
the ENZIAN score appears to be a good complement to the rASRM score for
morphologic description of deep endometriosis and planning of surgery, it is not
widely used. Recently the World Endometriosis Society (WES) published a
consensus statement in which they recommend that all women undergoing
surgery should have the rASRM classification completed, women with deep
endometriosis should additionally have ENZIAN completed, and women for
whom future fertility is a concern should additionally have the EFI
completed (244).
American Society for Reproductive Medicine Staging
The revised ASRM staging system, is based on the appearance, size, and
depth of peritoneal and ovarian implants; the presence, extent, and type of
adnexal adhesions; and the degree of cul-de-sac obliteration (179,201). In this
classification system, the morphology of peritoneal and ovarian implants
should be categorized as red (red, red-pink, and clear lesions), white (white,
yellow-brown, and peritoneal defects), and black (black and blue lesions),
702according to color photographs provided by ASRM.
This system reflects the extent of endometriotic disease but has considerable
intraobserver and interobserver variability (245,246). [4] The ASRM
classification for endometriosis is subjective and correlates poorly with pain
and fertility outcomes (175). Despite these important shortcomings the WES
recommends its use because of two important reasons: it is the most widely used
staging system in clinical practice and endometriosis research, and it is the
rASRM system (partially) incorporated in the EFI and ENZIAN.
Endometriosis Fertility Index
[5] The EFI staging system predicts non-IVF pregnancy rates after surgical
staging and treatment of endometriosis (Fig. 13-7) (177). The EFI is based on
historical and surgical factors. The historical factors are age, years of
infertility, and prior pregnancies. The surgical factors consist of the total
ASRM score, the ASRM endometriosis score, and the least function score,
which describes functionality of the fallopian tubes, fimbriae, and ovaries.
The EFI was designed specifically for infertility patients who have had surgical
staging and treatment of their disease. It is not intended to predict any aspect of
endometriosis associated pain. It is required that the male and female gametes are
sufficiently functional to enable attempts at non-IVF conception. Severe uterine
abnormality that is clinically significant is not included in the EFI. However,
when this condition is found it does need to be taken into account in predicting
pregnancy rates.
ENZIAN Classification
The ENZIAN staging system supplements the rASRM staging with a precise
description of the location and extent of deep endometriosis lesions and the
involvement of retroperitoneal structures or other organs.
The anatomic location of the deep endometriotic lesions is described in three
compartments: A = rectovaginal septum and vagina; B = sacrouterine ligament to
pelvic wall; and C = rectum and sigmoid colon. The depth of invasion is rated for
all compartments (grade 1 = invasion <1 cm; grade 2 = invasion 1 to 3 cm; grade
3 = invasion >3 cm). Deep endometriotic lesions outside the pelvis and invasion
of organs is registered separately: FA = adenomyosis; FB = bladder; FU =
intrinsic involvement of the ureter; FI = intestinal disease cranial to the
rectosigmoid junction; and FO = other locations, such as abdominal wall
endometriosis. The ENZIAN classification seems to be useful in planning
endometriosis surgery, but more research is needed on the correlation with pain or
infertility and clinical relevant outcomes.
Spontaneous Evolution
Endometriosis appears to be a progressive disease in a significant proportion
(30% to 60%) of patients. During serial observations, deterioration (47%),
improvement (30%), or elimination (23%) was documented over a 6-month
period (247,248). In another study, endometriosis progressed in 64%, improved in
27%, and remained unchanged in 9% of patients over 12 months (249). A third
study of 24 women reported 29% with disease progression, 29% with disease
regression, and 42% with no change over 12 months. Follow-up studies in both
baboons and women with spontaneous endometriosis over 24 months
demonstrated disease progression in all baboons and in 6 of 7 women (250–252).
Several studies reported that subtle lesions and typical implants may represent
younger and older types of endometriosis, respectively. In a cross-sectional study,
the incidence of subtle lesions decreased with age (253). This finding was
confirmed by a 3-year prospective study that reported that the incidence, overall
pelvic area involved, and volume of subtle lesions decreased with age, but in
typical lesions, these parameters and the depth of infiltration increased with age
(7). Remodeling of endometriotic lesions (transition between typical and subtle
subtypes) is reported to occur in women and in baboons, indicating that
endometriosis is a dynamic condition (254,255). Several studies in women,
cynomolgus monkeys, and rodents showed that endometriosis is ameliorated after
pregnancy (255–258).
The characteristics of endometriosis are variable during pregnancy, and
lesions tend to enlarge during the first trimester but regress thereafter (259).
Studies in baboons revealed no change in the number or surface area of
endometriosis lesions during the first two trimesters of pregnancy (260). These
results do not exclude a beneficial effect that may occur during the third trimester
or in the immediate postpartum period. Establishment of a “pseudopregnant state”
with exogenously administered estrogen and progestins is based on the belief that
symptomatic improvement may result from decidualization of endometrial
implants during pregnancy (261). This hypothesis is not substantiated, and it is
possible that amenorrhea can explain the beneficial effect of pregnancy and
lactation on endometriosis-associated pain symptoms.
MANAGEMENT
Primary Prevention
No strategies to prevent endometriosis are uniformly successful. A reduced
incidence of endometriosis was reported in women who engaged in aerobic
activity from an early age, but the possible protective effect of exercise was not
investigated thoroughly (48). There is insufficient evidence that OC use offers
704protection against the development of endometriosis. One report showed an
increased risk for endometriosis in a select population of women taking OCs,
possibly explained by the observation that dysmenorrhea as a reason to initiate
estroprogestins is significantly more common in women with endometriosis than
in women without the disease (262,263). OCs inhibit ovulation, substantially
reduce the volume of menstrual flow, and may interfere with implantation of
refluxed endometrial cells, but the hypothesis of recommending OCs for primary
prevention of endometriosis is not sufficiently substantiated (264). Although the
risk of endometriosis appears reduced during OC use, it is possible that this effect
results from postponement of surgical evaluation caused by temporary
suppression of pain symptoms (265). Confounding by selection and indication
biases may explain the trend toward an increase in risk of endometriosis observed
after discontinuation, but further clarification is needed (265).
FIGURE 13-7 Endometriosis Fertility Index. (From Adamson D, Pasta D. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil Steril
2010;94:1609–1615.)
Principles of Treatment
Treatment of endometriosis must be individualized, taking into consideration the clinical problem in its entirety, including the impact of the disease and the effect of its treatment on quality of life. Evidence-based recommendations that are continuously updated can be found in the ESHRE guidelines for the clinical management of endometriosis (1).
In most women with endometriosis, preservation of reproductive function
is desirable (1). Many women with endometriosis have pain and infertility at
the same time or may desire children after sufficient pain relief, which
complicates the choice of treatment. Endometriosis surgery should be
considered as reproductive surgery, defined by the World Health Organization
(WHO) as “all surgical procedures carried out to diagnose, conserve, correct
and/or improve reproductive function” (266). The least invasive and least
expensive approach that is effective with the least long-term risks should be
chosen (1). Symptomatic endometriosis patients can be treated with
analgesics, hormones, surgery, assisted reproduction, or a combination of
these modalities (1). Regardless of the clinical profile (infertility, pain,
asymptomatic findings), treatment of endometriosis may be justified because
endometriosis appears to progress in 30% to 60% of patients within a year of
diagnosis and it is not possible to predict in which patients it will progress
(249). Elimination of the endometriotic implants by surgical or medical treatment
often provides only temporary relief. In addition to eliminating the endometriotic
lesions, the goal should be to treat the sequelae (pain and infertility) often
associated with this disease and to prevent recurrence of endometriosis (1).
Endometriosis is a chronic disease and the recurrence rate is high after both
hormonal and surgical treatment (1).
Treatment of extragenital endometriosis will depend on the site. If
complete excision is possible, this is the treatment of choice; when this is not
possible, long-term medical treatment is necessary using the same principles
of medical treatment for pelvic endometriosis (1).
It is important to involve the patient in all decisions, to be flexible in
considering diagnostic and therapeutic approaches, and to maintain a good
relationship. It may be appropriate to seek advice from more experienced
colleagues or to refer the patient to a center with the necessary expertise to offer
treatments in a multidisciplinary context, including advanced laparoscopic
surgery and laparotomy (1,267). Because the management of severe or deep
endometriosis is complex, referral is strongly recommended when disease of such
707severity is suspected or diagnosed (1).
Treatment of Endometriosis-Associated Pain
Pain may persist despite seemingly adequate medical or surgical treatment of
the disease. A multidisciplinary approach involving a pain clinic and
counseling should be considered early in the treatment plan. The least
invasive and least expensive approach that is effective should be used (1).
Surgical Treatment
Depending on the severity of disease, diagnosis and removal of endometriosis
should be performed simultaneously at the time of surgery, provided that
preoperative consent was obtained (1,268–271). The goal of surgery is to excise
all visible endometriotic lesions and associated adhesions—peritoneal lesions,
ovarian cysts, deep rectovaginal endometriosis—and to restore normal
anatomy (1). Laparoscopy is preferred over laparotomy because the two
techniques are equally effective and laparoscopy is associated with quicker
recovery, better cosmesis, less postoperative pain, decreased costs, lower
morbidity, and fewer postoperative adhesions (1). Laparotomy is only
indicated in the rare cases of advanced-stage disease where laparoscopy is
impossible.
Conservative Surgery
Peritoneal Endometriosis
Endometriosis lesions can be removed during laparoscopy by surgical
excision with scissors, bipolar coagulation, or laser methods (CO2 laser,
potassium-titanyl-phosphate laser, or argon laser). Some surgeons claim that the
CO2 laser is superior because it causes only minimal thermal damage, but no
evidence is available to show the superiority of one technique over another.
Surgical ablation of peritoneal endometriosis is considered equally effective as
surgical excision. However, surgical excision of lesions could be preferred
because it allows histologic analysis and confirmation of endometriosis.
Ovarian Endometriosis
Superficial ovarian lesions can be vaporized. The surgical management of
pain associated with ovarian endometriotic cysts is controversial. The most
common procedures for the treatment of ovarian endometriomas are either
excision of the cyst wall or drainage and ablation of the cyst wall. During
excision, the ovarian endometrioma is aspirated, followed by incision and
removal of the cyst wall from the ovarian cortex with maximal preservation of
708normal ovarian tissue. During drainage and ablation, the ovarian endometrioma is
aspirated and irrigated. Its wall can be inspected with ovarian cystoscopy for
intracystic lesions, and it is vaporized to destroy the mucosal lining of the cyst.
According to a systematic review, there is good evidence that excisional
surgery for endometriomas with a diameter of 3 cm provides a more favorable
outcome than drainage and ablation with regard to the recurrence of the
endometrioma, recurrence of pain symptoms, and in women who were previously
subfertile or had subsequent spontaneous pregnancy (272). Laparoscopic excision
of the cyst wall of the endometrioma was associated with a reduced recurrence
rate of the symptoms of dysmenorrhea (odds ratio [OR] 0.15; 95% CI, 0.06–
0.38), dyspareunia (OR 0.08; 95% CI, 0.01–0.51), and nonmenstrual pelvic pain
(OR 0.10; 95% CI, 0.02–0.56), a reduced rate of recurrence of the endometrioma
(OR 0.41; 95% CI, 0.18–0.93), and with a reduced requirement for further
surgery (OR 0.21; 95% CI, 0.05–0.79) than surgery to ablate the endometrioma.
For those women subsequently attempting to conceive, it was associated with an
increased spontaneous pregnancy rate in women who had documented prior
infertility (OR 5.21; 95% CI, 2.04–13.29).
Based on this evidence, the ESHRE guideline recommends cystectomy over
drainage and coagulation because a cystectomy reduces endometriosisassociated pain and has a lower recurrence rate. In case of very large
endometriomas where excision is technically difficult without removing a large
part of the ovary, a three-step procedure (marsupialization and rinsing followed
by hormonal treatment with GnRH analogs and cyst wall electrocoagulation or
laser vaporization 3 months later) can be considered (1,273).
It is possible that the surgical techniques used to treat ovarian endometriotic
cysts may influence postoperative adhesion formation and/or ovarian function. In
a randomized study comparing surgical methods to achieve ovarian hemostasis
after laparoscopic endometriotic ovarian cystectomy, closure of the ovary with an
intraovarian suture resulted in a lower rate and extension of postsurgical ovarian
adhesions at 60 to 90 days follow-up when compared to only bipolar coagulation
on the internal ovarian surface (274).
Deep Endometriosis
Deep endometriosis is usually multifocal and complete surgical excision must
be performed in a one-step surgical procedure in order to avoid more than
one surgery, provided the patient is fully informed (1,173,269). Because
management of deep endometriosis is complex, referral to a center with sufficient
expertise to offer all available treatments in a multidisciplinary approach is
strongly recommended (1). Surgical management is only for symptomatic deep
endometriosis. Asymptomatic patients must not undergo surgery, except for cases
709with complete obstruction of a ureter resulting in asymptomatic loss of renal
function. Progression of the disease and appearance of specific symptoms rarely
occurred in patients with asymptomatic rectovaginal endometriosis (270). When
surgical treatment is decided, the treatment must be radical with excision of all
lesions (1). It is difficult to perform randomized studies to detect the best surgical
technique to treat deep endometriosis because these severe cases are all managed
individually and not all surgeons are familiar with all treatment options (1).
Complete excision while preserving the uterus and ovarian tissue might include
the resection of the uterosacral ligaments, the resection of the upper part of the
posterior vaginal wall, and urologic and bowel operations.
The patients’ surgical agreement must be obtained preoperatively to
perform this difficult and high-risk procedure, especially in cases of expected
or possible bowel or urologic surgery. Preoperative imaging is necessary to
assess bowel and urologic impact of deep endometriosis. As endometriosis
sometimes involves nongynecologic organs (i.e., the bowel, the urinary tract, or
pelvic bones), other surgical specialists should be consulted as appropriate. These
severe cases should be handled in centers with special expertise. Preoperative
intestinal preparation may be recommended. Placement of ureteric catheters may
facilitate the excision of periureteral endometriosis to facilitate ureterolysis and
end-to-end ureteral reanastomosis that may be needed in cases of infiltrative
periureteral endometriosis. The pattern of pain in endometriosis is complicated
and pain does not always respond to treatment, so consultation with pain
specialists may be useful.
In patients with severe endometriosis, it is common clinical practice that
surgical treatment be preceded by a 3-month course of medical treatment
(194). It is believed that this facilitates surgery by reducing inflammation and
vascularization of lesions. The role of preoperative hormonal treatment was
evaluated in a Cochrane review that concluded that there was no evidence of a
benefit of preoperative medical therapy on the outcome of surgery. Therefore,
although common clinical practice, the ESHRE guideline does not recommend
preoperative hormonal therapy.
Surgical treatment of bladder endometriosis is usually in the form of excision
of the lesion and primary closure of the bladder wall. Removal of full-thickness
bladder detrusor endometriosis entails excision of the bladder dome or posterior
wall, generally well above the trigone. Transurethral resection is contraindicated.
Ureteral lesions may be excised after stenting the ureter. In the presence of
intrinsic lesions or significant obstruction, segmental excision with end-to-end
anastomosis or reimplantation with antireflux vesicoureteral plasty may be
necessary (275).
Surgical excision of deep rectovaginal and rectosigmoidal endometriosis is
710difficult and can be associated with major complications such as bowel
perforations with resulting peritonitis (276). It is debated whether this type of
endometriosis is best treated by shaving, conservative excision, or resection
reanastomosis, by laparoscopy and laparotomy, or laparoscopically assisted
vaginal technique (277). Appendicular endometriosis is usually treated by
appendectomy.
In a randomized study comparing colorectal resection for endometriosis by
laparoscopy or laparotomy, clinical outcome was similar with respect to
dyschezia, bowel pain and cramping, and dysmenorrhea and dyspareunia, but
laparoscopy was associated with less blood loss, fewer complications, and a
higher pregnancy rate than laparotomy (278).
There are very few methodologically valid studies evaluating clinical outcome
after surgery for deep endometriosis with colorectal extension, as demonstrated in
a systematic review (279). In a review on the clinical outcome of surgical
treatment of deep endometriosis with colorectal involvement, most of the 49
reviewed studies included complications (94%) and pain (67%); few studies
reported recurrence (41%), fertility (37%), and quality of life (10%); only 29%
reported (loss of) follow-up. Of 3,894 patients, 71% underwent bowel resection
and anastomosis, 10% had full-thickness excision, and 17% were treated with
superficial surgery. Comparison of clinical outcome between different surgical
techniques was not possible. Postoperative complications were present in 0% to
3% of the patients. Although pain improvement was reported in most studies, pain
evaluation was patient based in less than 50% (visual analog scale [VAS] in only
18%). Although quality of life was improved in most studies, prospective data
were available for only 149 patients. Pregnancy rates were 23% to 57% with a
cumulative pregnancy rate of 58% to 70% within 4 years. The overall
endometriosis recurrence rate in studies (longer than 2 years follow-up) was 5%
to 25%, with most of the studies reporting 10%.
Because high quality prospective studies reporting standardized and welldefined clinical outcomes after surgical treatment of deep endometriosis with
long-term follow-up are needed, the Consensus On Recording Deep
Endometriosis Surgery (CORDES) statement was published in 2016 (280). This
consensus statement provides definition and standards for recording deep
endometriosis surgery and outcome reporting in clinical trials on the surgical
management of deep endometriosis.
Adhesiolysis
Endometriosis is often associated with pelvic adhesions, which can be very
extensive and result in severe distortion of the pelvic anatomy. The removal of
endometriosis-related adhesions (adhesiolysis) should be performed carefully and
711focused at restoration of the normal anatomy. However, adhesions lysed at
surgery can form again. Cutting, surgical denudation, ischemia, desiccation, or
abrasion can cause peritoneal trauma during surgery and the subsequent healing
mechanism in the peritoneal cavity can result in adhesion formation between
damaged serosal surfaces. Minimally invasive techniques such as laparoscopy
reduce the risk of adhesion formation but do not eliminate it entirely.
Several barrier agents have been tested for adhesion prevention during surgery.
The ESHRE guideline acknowledges that clinicians can consider the use of
oxidized regenerated cellulose and potentially other barrier agents. However, a
recent systematic review including 18 randomized controlled trials (RCTs)
with a total of 1,262 enrolled women found no effects of any barrier agent
used during pelvic surgery on either pain or fertility outcomes (281). Lowquality evidence suggests that oxidized regenerated cellulose, expanded
polytetrafluoroethylene and sodium hyaluronate with carboxymethylcellulose
may all be more effective than no treatment in reducing the incidence of adhesion
formation following pelvic surgery.
Based on this systematic review, routine use of barrier agents to prevent
postoperative adhesions after pelvic surgery can be considered but cannot be
recommended.
Interruption of Pelvic Nerve Pathways
Surgical interruption of pelvic nerve pathways has been suggested as an
additional procedure to conservative surgery for endometriosis. Two techniques
have been suggested for endometriosis-associated pain: laparoscopic uterine
nerve ablation (LUNA) and presacral neurectomy (PSN). The effectiveness of
LUNA and PSN was evaluated in several clinical trials and data of six RCTs were
analyzed in a Cochrane review (282). Based on the systematic review it can be
concluded that laparoscopic excision or ablation of endometriosis should not be
combined with LUNA because this procedure offers no additional benefit. PSN is
effective as an additional procedure, but it requires a high degree of surgical skill
and is associated with increased adverse effects such as bleeding, constipation,
urinary urgency, and less pain during the first stage of labor (1).
Outcome of Conservative Surgery
The outcome of surgical therapy in patients with endometriosis and pain is
influenced by many psychological factors relating to personality, depression,
and marital and sexual problems. It is difficult to evaluate scientifically the
objective effect of different surgical approaches because the extirpation and
destruction of the pathologic tissue can impact the results as can surgery per
se, the doctor–patient relationship, complications, and other factors. There is
712a significant placebo response to surgical therapy: diagnostic laparoscopy
without complete removal of endometriosis may alleviate pain in 50% of
patients (283–285). Similar results were reported using oral placebos (286).
Although some reports claimed pain relief with laser laparoscopy in 60% to 80%
of patients with very low morbidity, none were prospective or controlled or
allowed a definitive conclusion regarding treatment efficacy (194,287–290). The
longstanding effect of surgery on pain is difficult to evaluate because the followup time is too short, usually just a few months. The major shortcoming of surgical
treatment in endometriosis-related pain is the lack of prospective randomized
studies with sufficient follow-up time to draw clear clinical conclusions.
In a systematic review assessing the efficacy of laparoscopic surgery in the
treatment of pelvic pain associated with endometriosis and including five
randomized controlled studies, meta-analysis demonstrated an advantage of
laparoscopic surgery when compared to diagnostic laparoscopy only at 6 months
(OR 5.72; 95% CI, 3.09–10.60; 171 participants, three trials) and 12 months (OR
7.72; 95% CI, 2.97–20.06; 33 participants, one trial) after surgery (291). Few
women diagnosed with severe endometriosis were included in the meta-analysis
and any conclusions from this meta-analysis regarding treatment of severe
endometriosis should be made with caution. It was not possible to draw
conclusions from the meta-analysis in which specific laparoscopic surgical
intervention was most effective (291).
The extent and duration of the therapeutic benefit of surgery for
endometriosis-related pain are poorly defined, and the expected benefit is
operator dependent (292). In a systematic review based on three randomized
controlled studies, the absolute increased benefit from destruction of lesions
compared with diagnostic only operation in terms of proportion of women
reporting pain relief was between 30% and 40% after short follow-up periods
(292). The pain relief tended to decrease with time, and the reoperation rate,
based on long-term follow-up studies, was as high as 50% (292). In most case
series on excisional surgery for rectovaginal endometriosis, substantial short-term
pain relief was experienced by approximately 70% to 80% of the patients who
continued the study. At 1-year follow-up, approximately 50% of the women
needed analgesics or hormonal treatments (292). Medium-term recurrence of
lesions was observed in approximately 20% of the cases, and approximately 25%
of the women underwent repetitive surgery (292). It appears that pain recurrence
and reoperation rates after conservative surgery for symptomatic endometriosis
are high and probably underestimated (292).
Prevention of Recurrence After Conservative Surgical Treatment
The addition of postoperative hormonal therapy to conservative surgery for
713endometriosis has been suggested for two main reasons. First, short-term
postoperative hormonal therapy (up to 6 months after the operation) could
potentially improve the outcomes of the operation through their effect on any
residual endometriosis. Secondly, long-term hormonal therapy after surgery could
be prescribed for secondary prevention: suppression of ovarian function and
menstruation could prevent the development of new lesions.
Systemic Medical Therapy
In a systematic review published in 2004 to determine the effectiveness of
systemic medical therapies used for hormonal suppression before or after surgery
for endometriosis, or before and after surgery for the eradication of endometriosis,
improvement of symptoms, pregnancy rates, and overall tolerability, by
comparing them with no treatment or placebo, 11 trials were included (293). Five
trials compared postsurgical medical therapy with surgery alone (without
medical therapy) and assessed the outcomes of pain recurrence, disease
recurrence, and pregnancy rates (294–297). There was no statistically
significant reduction in pain recurrence at 12 months (relative risk [RR] 0.76;
95% CI, 0.52–1.10), but the difference at 24 months approached statistical
significance (RR 0.70; 95% CI, 0.47–1.03). There was no statistically
significant difference between the use of these medical therapies after
surgery compared to surgery alone with regard to disease recurrence (RR
1.02; 95% CI, 0.27–3.84) or pregnancy rates (RR 0.78; 95% CI, 0.50–1.22).
Postsurgical medical therapy was compared to surgery plus placebo in three
studies (295,298,299). There was no difference between medical therapy and
placebo with regard to the measures for pain; multidimensional pain score (WMD
−0.40; 95% CI, −2.15–1.35); linear scale score (0.10; 95% CI, −2.24–2.44); or
change in pain (−0.40; 95% CI, −1.48–0.68). There was no difference between
medical therapy and placebo for pregnancy rates (RR 1.05; 95% CI, 0.44–2.51) or
total AFS scores (WMD −2.10; 95% CI, −4.56–0.36). There was no significant
difference between preoperative hormonal suppression and postoperative
hormonal suppression for the outcome of pain in one trial (300).
These results were confirmed in another RCT with 5 years of follow-up,
showing that GnRH analog treatment with triptorelin depot 3.75 intramuscular
after operative laparoscopy for stages III and IV endometriosis was comparable to
placebo injections with respect to time to relapse of endometrioma, pain
recurrence, and time to pregnancy (301).
There is circumstantial evidence that regular postoperative use of OCs
effectively prevents endometrioma recurrence (302). In a prospective
controlled cohort study with a median follow-up of 28 months after laparoscopic
excision of ovarian endometriomata, the 36-month cumulative proportion of
714subjects free from endometrioma recurrence was 94% in women who always used
cyclic oral contraception compared with 51% in those who never used it (p
<0.001; adjusted incidence rate ratio [IRR] 0.10; 95% CI, 0.04–0.24) (302).
Some randomized controlled studies suggest that postoperative hormonal
treatment can be useful in delaying the recurrence of endometriosis and/or pelvic
pain. One study supported the long-term postoperative use of OCs to reduce the
frequency and the severity of recurrent endometriosis-related dysmenorrhea
(303). The prevention of endometriosis-related pain recurrence by postoperative
long-term (24 months) cyclic and continuous administration of OCs was
compared to no treatment in women after surgery for ovarian endometrioma. A
significant reduction in recurrence rate and VAS scores for dysmenorrhea was
evident in the continuous users versus the other groups at 6 months, and in cyclic
users versus nonusers at 18 months postoperatively. No significant differences in
recurrence rate and VAS scores for dyspareunia and chronic pelvic pain were
demonstrated among the groups. The increase of VAS scores for dysmenorrhea,
dyspareunia, and chronic pelvic pain during the postoperative follow-up of 6 to
24 months was significantly higher in nonusers than in the users (303).
In a second study, long-term cyclic and continuous postoperative use of
OCs was shown to effectively reduce and delay endometrioma recurrence
(304). The crude recurrence rate within 24 months was significantly lower in
cyclic (14.7%) and continuous users (8.2%) compared with nonusers (29%). The
recurrence-free survival was significantly lower in nonusers compared with cyclic
and continuous users. The mean recurrent endometrioma diameter at first
observation and the mean diameter increase every 6 months of follow-up were
significantly lower in cyclic and continuous users compared with nonusers,
whereas no significant differences between cyclic users and continuous users
in terms of endometrioma recurrence were demonstrated (304).
In a third study, women who underwent conservative pelvic surgery for
symptomatic endometriosis stages III and IV (r-AFS) were treated postoperatively
during 6 months with either placebo, GnRH agonist (triptorelin or leuprorelin,
3.75 mg every 28 days), continuous estroprogestin (ethinylestradiol, 0.03 mg plus
gestodene, 0.75 mg) or dietary therapy (vitamins, minerals salts, lactic ferments,
fish oil) (305). At 12 months after surgery, patients treated with postoperative
hormonal suppression therapy showed a lower VAS score for dysmenorrhea than
patients in the other groups. Hormonal suppression therapy and dietary
supplementation were equally effective in reducing nonmenstrual pelvic pain.
Postoperative medical and dietary therapy allowed a better quality of life
when compared to placebo treatment (305).
In a systematic review to determine whether postoperative use of an
levonorgestrel intrauterine system in women with endometriosis improves pain
715symptoms associated with menstruation and reduces recurrence compared with
surgery only, placebo, or systemic hormones, one small RCT was identified
showing a statistically significant reduction in the recurrence of painful periods in
the levonorgestrel intrauterine system treated group compared with the control
group receiving a GnRH agonist (OR 0.14; 95% CI, 0.02–0.75) (306,307). The
proportion of women who were satisfied with their treatment was higher in the
levonorgestrel intrauterine system treated group than in the control group, but this
difference did not reach statistical difference (OR 3.00; 95% CI, 0.79–11.44)
(306,307). In another small randomized trial, postsurgical treatment with either
levonorgestrel intrauterine system or depot MPA for 3 years indicated symptom
control and recurrence were comparable, but compliance and change in bone
mineral density were better in the levonorgestrel intrauterine system treated group
than in the depot MPA group (308).
Based on all this evidence, it can be concluded that there is no strong evidence
that short-term postoperative hormonal therapy improves the outcome of
conservative surgery for endometriosis, hence it is not recommended in the
ESHRE guideline. However, there is a role for postoperative hormonal therapy
for secondary prevention of endometriosis. There is no good evidence to
recommend one intervention and the ultimate choice will be influenced by patient
preferences, side effects, and costs. The ESHRE guideline recommends the use
of postoperative hormonal therapy in two indications: (1) after cystectomy
for an endometrioma in women not immediately seeking conception; and (2)
after conservative surgery for endometriosis, for at least 18 to 24 months, for
secondary prevention of endometriosis-associated dysmenorrhea.
Radical Surgery: Oophorectomy and Hysterectomy
Radical procedures such as oophorectomy or total hysterectomy are
indicated only in severe situations and can be performed laparoscopically or
by laparotomy. Women aged 30 years or younger at the time of hysterectomy for
endometriosis-associated pain are more likely than older women to have residual
symptoms, to report a sense of loss, and to report more disruption from pain in
different aspects of their lives (309). If a hysterectomy is performed, all visible
endometriotic tissue should be removed. Although associated with improved pain
relief and a reduced chance of future surgery, a bilateral salpingo-oophorectomy
(BSO) in young women should be considered in only the most severe or recurrent
cases (310,311). Resection is an effective treatment for rectovaginal
endometriosis, in combination with hysterectomy (312).
Medical Treatment of Endometriosis-Associated Pain
If the patient desires treatment of pain symptoms that are suggestive of
716endometriosis in the absence of a definitive diagnosis, empirical treatment is
appropriate and includes counseling, analgesia, and progestins or combined
OCs. Before starting empirical treatment, other causes of pelvic pain symptoms
should be ruled out, as far as possible. It is unclear whether OCs should be
taken in a conventional, continuous, or tricycle regimen. A GnRH agonist
may be taken, but is not recommended, because this class of drug is more
expensive and associated with more side effects and concerns about bone density
than OCs (1).
Primary Dysmenorrhea
Analgesics
Women suffering from dysmenorrhea are treated with analgesics; many women
treat themselves with over-the-counter oral analgesics. Primary dysmenorrhea is
defined as menstrual pain without organic pathology, based on physical
examination alone, and it can be argued that some women with so-called primary
dysmenorrhea probably have endometriosis (313). In a systematic review, it was
concluded that nonsteroidal anti-inflammatories (NSAIDs) were more
effective than placebo for relief of primary dysmenorrhea, but there was
insufficient evidence to suggest whether any individual NSAID was more
effective than another (314). In another review, selective cyclooxygenase-2
inhibitors rofecoxib and valdecoxib were as effective as naproxen and more
effective than placebo for the treatment of primary dysmenorrhea (315). Concerns
were raised about the safety of these medications, and its manufacturers withdrew
rofecoxib from the market.
According to another systematic review based on two relatively small, RCTs
comparing paracetamol and coproxamol with placebo, coproxamol (paracetamol
650 mg and dextropropoxyphene 65 mg) but not paracetamol (500 mg 4 times
daily) was more effective than placebo in reducing primary dysmenorrhea (315).
This observation may be explained by the suboptimal dosage of paracetamol
used. A small randomized trial demonstrated that paracetamol (acetaminophen)
1,000 mg four times daily was superior to placebo for the treatment of primary
dysmenorrhea (316).
Oral Contraceptives
There is a paucity of information for the use of modern OCs for primary
dysmenorrhea. A Cochrane review suggested that first- and second-generation
OCs with 50 μg or more estrogen may be more effective than placebo treatment
for dysmenorrhea. It concluded that the studies included for analysis were of poor
quality and heterogeneous so that no recommendation could be made regarding
the efficacy of modern, lower-dose OCs (evidence level 1a) (317). An RCT
717comparing a low-dose OC containing 20 μg ethinyl estradiol and 100 μg
levonorgestrel with placebo showed better pain relief in adolescent girls with
dysmenorrhea using the OC than placebo (318).
There is some evidence in general populations that OCs can effectively treat
dysmenorrhea (319). OCs have the advantage of long-term safety; hence they can
be used indefinitely in low-risk women. In clinical practice, when they are used
for menstrual pain, they may be taken continuously or with one pill-free week per
three cycles to reduce the number of periods or avoid them altogether. There is no
direct comparison of these options with the conventional approach.
Other Treatments
Several Cochrane reviews and one clinical evidence review suggest that other
treatment modalities that might be helpful in primary dysmenorrhea include
supplemental thiamine or vitamin E, high-frequency transcutaneous nerve
stimulation, topical heat and herbal remedy toki-shakuyaku-san. They suggest that
treatment modalities with unknown benefits are vitamin B12, fish oil, magnesium,
acupuncture, other herbal remedies, and behavioral interventions, and that spinal
manipulation is unlikely to be beneficial (317,319–321).
Treatment of Endometriosis-Associated Pain
Nonsteroidal Anti-Inflammatory Drugs
Considering that endometriosis is a chronic inflammatory disease, antiinflammatory drugs would appear to be effective for treatment of endometriosisrelated dysmenorrhea. Although NSAIDs are used extensively and are often the
first-line therapy for reduction of endometriosis-related pain, the analgesic effect
of NSAIDs was not studied extensively. Only one small, double-blind, placebocontrolled, four-period, crossover clinical study was published (322). This study
claimed complete or substantial pain relief of endometriosis-related dysmenorrhea
in 83% of cases treated with naproxen compared with 41% in cases treated with
placebo. A different analysis of the data from the same study by the Cochrane
Collaborative Network did not confirm a positive effect of naproxen on pain
relief (OR 3.27; 95% CI, 0.61–17.69) in women with endometriosis (323).
There was inconclusive evidence to indicate whether women taking NSAIDs
(naproxen) were less likely to require additional analgesia (OR 0.12; 95% CI,
0.01–1.29) or to experience side effects (OR 0.46; 95% CI, 0.09–2.47) when
compared to placebo (323).
Endometriosis-related pain is nociceptive, but persistent nociceptive input from
endometriotic lesions leads to central sensitization manifested by somatic
hyperalgesia and increased referred pain (324). The potential effectiveness of
718NSAIDs in the reduction of endometriosis-related pain may be explained by a
local antinociceptive effect and a reduced central sensitization in addition to the
anti-inflammatory effect. NSAIDs have significant side effects, including gastric
ulceration and possible inhibition of ovulation. PGs are involved in the follicle
rupture mechanism at ovulation, which is why women who wish to become
pregnant should not take NSAIDs at the time of ovulation (325).
Hormonal Treatment
Effect of Hormonal Treatment on Pain
Because estrogen is known to stimulate the growth of endometriosis, hormonal therapy is designed to suppress estrogen synthesis, thereby inducing atrophy of ectopic endometrial implants or interrupting the cycle of stimulation and bleeding (1). Implants of endometriosis react to gonadal steroid hormones in a manner similar, but not identical, to normally stimulated ectopic endometrium. Ectopic endometrial tissue displays histologic and biochemical differences from normal ectopic endometrium in characteristics such as glandular activity (proliferation, secretion), enzyme activity (17β-hydroxysteroid dehydrogenase), and steroid (estrogen, progestin, and androgen) hormone receptor levels. Withdrawal of estrogen stimulation causes cellular inactivation and degeneration of endometriotic implants but not their disappearance.
There is strong evidence that suppression of ovarian function for 6 months reduces pain associated with endometriosis. [6] Combined OCs danazol, gestrinone, medroxyprogesterone acetate, and GnRH agonists are all equally effective but their side effects and cost profiles differ (1). Pain relief may be of short duration, presumably because endometriosis and endometriosisassociated pain recur after the cessation of medical treatment. The use of DES, methyltestosterone, or other androgens is no longer advocated because they lack efficacy, have significant side effects, and pose risks to the fetus if pregnancy occurs during therapy. A new generation of aromatase inhibitors, oral GnRH antagonists, estrogen receptor modulators, and progesterone antagonists (PRAs) may offer new hormonal treatment options.
Hormonal Treatment for Pain From Rectovaginal Endometriosis
Surgical treatment may reduce the pain associated with rectovaginal endometriosis, but it is associated with a high risk of morbidity and major complications. The effect of medical treatment in terms of pain relief in women with rectovaginal endometriosis appears to be substantial (326). In a systematic review including 217 cases of medically treated rectovaginal endometriosis, the antalgic effect of the considered medical therapies (vaginal danazol, GnRH agonist, progestin, and estrogen–progestin combinations used transvaginally, transdermally, or orally) for the entire treatment period (from 6 to 12 months) was 60% to 90%, with patients reporting considerable reduction or complete relief from pain symptoms, with the exception of when an aromatase inhibitor was used alone (326).
Oral Contraceptives
Although OCs are effective in inducing a decidualized endometrium, the estrogenic component in OCs may stimulate endometrial growth and increase
pelvic pain in the first few weeks of treatment. The long-term significance of this
effect is undetermined. OCs are less costly than other treatments and may be
helpful in the short-term management of endometriosis with potential long-term
benefits in some women.
The use of cyclic OCs may provide prophylaxis against the development or
recurrence of endometriosis. Estrogens in OCs may stimulate the proliferation
of endometriosis. The reduced menstrual bleeding that often occurs in women
taking OCs may be beneficial to women with prolonged, frequent menstrual
bleeding, which is a known risk factor for endometriosis (48).
Further research is warranted to assess the effect of low-dose OCs in
preventing endometriosis and treating associated pain, because the evidence for
its efficacy is limited. In a systematic review to assess the effects of OC’s in
comparison with other treatments for painful symptoms of endometriosis in
women of reproductive age, only one study met the inclusion criteria (all truly
RCTs of the use of OCs in the treatment of women of reproductive age with
symptoms ascribed to the diagnosis of endometriosis and made visually at
surgical procedure were included) (327,328). In this study, a total of 57 women
were allocated to two groups to compare an OC to a GnRH analog (328).
Methods of randomization and allocation concealment were unclear and the
analog group became amenorrheic during the treatment period of 6 months, while
women in the OC group reported a decrease in dysmenorrhea (327,328). No
evidence of a significant difference between the two groups was observed in
terms of dysmenorrhea at 6 months follow-up after stopping treatment (OR 0.48;
95% CI, 0.08–2.90). Some evidence for a decrease in dyspareunia was found at
the end of treatment in women in the GnRH analog group; although no evidence
of a significant difference in dyspareunia was observed at the end of the 6 months
follow-up (OR 4.87; 95% CI, 0.96–24.65). According to these data, there is no
evidence of a difference in outcomes between the OC studied and GnRH analog
in the treatment of endometriosis-associated painful symptoms (327). The lack of
studies with larger sample sizes or studies focusing on other comparable
treatments is concerning, and further research is needed to evaluate the role of
OCs in managing symptoms associated with management of endometriosis (327).
720In a double-blind, randomized, placebo-controlled trial, patients with suspected
or surgically proven endometriosis were randomly assigned to receive either
monophasic OC (ethinylestradiol plus norethisterone) or placebo during four
cycles (329). Total dysmenorrhea scores assessed by verbal rating scale were
significantly decreased at the end of treatment in both groups. From the first cycle
through the end of treatment, dysmenorrhea in the OC group was significantly
milder than in the placebo group. The volume of ovarian endometrioma was
significantly decreased in the OC group but not in the placebo group (329).
Continuous Contraceptives
The treatment of endometriosis with continuous low-dose monophasic OCs (one pill per day for 6 to 12 months) was originally used to induce pseudopregnancy caused by the resultant amenorrhea and decidualization of endometrial tissue (261). The concept was to induce an adynamic endometrium through elimination
of the normal cyclic hormonal changes characteristic of the menstrual cycle (330).
This induction of a pseudopregnancy state with combination OC pills is effective
in reducing dysmenorrhea and pelvic pain. The subsequent amenorrhea induced
by OCs could decrease the risk for disease progression by preventing or reducing
(retrograde) menstruation. Pathologically, OC use is associated with
decidualization of endometrial tissue, necrobiosis, and possibly absorption of the
endometrial tissue (331). There is no convincing evidence that medical therapy
with OCs offers definitive therapy. Instead, the endometrial implants survive
the induced atrophy and, in most patients, reactivate after termination of
treatment.
Any low-dose OC containing 20 to 35 lg of ethinyl estradiol used
continuously can be used for the management of endometriosis. The
objective of the treatment is the induction of amenorrhea, which should be
continued for 6 to 12 months. Continuous or extended cyclic use of OCs is well
tolerated when compared to the cyclic use of OCs for contraceptive purposes
(332). In an RCT of women with recurrent moderate or severe pelvic pain after
unsuccessful conservative surgery for symptomatic rectovaginal endometriosis,
continuous treatment with oral ethinyl E2, 0.01 mg plus cyproterone acetate, 3
mg per day, or norethindrone acetate, 2.5 mg per day during 12 months resulted
in substantially reduced dysmenorrhea, deep dyspareunia, nonmenstrual pelvic
pain, and dyschezia scores without major between-group differences in patient
satisfaction rates (62% and 73%, respectively) (333).
Table 13-1 Medical Treatment of Endometriosis-Associated Pain: Effective Regimens
(Usual Duration: 6 Months)
721In a patient preference cohort study to evaluate the efficacy and tolerability of a
contraceptive vaginal ring (supplying 15 μg of ethinyl E and 120 μg per day of
etonogestrel) and transdermal patch (delivering 20 μg of ethinyl E and 150 μg per
day norelgestromin) in the treatment of women with recurrent moderate or severe
pelvic pain after conservative surgery for symptomatic endometriosis and
endometriosis-associated pain, patients who preferred the ring were significantly
more likely to be satisfied and to comply with treatment than those who chose the
patch (264). Both systems were associated with poor bleeding control when used
continuously.
Progestins
Progestins may exert an antiendometriotic effect by causing initial
decidualization of endometrial tissue followed by atrophy. Progestins can be
considered as the first choice for the treatment of endometriosis because they
are as effective as danazol or GnRH analogs and have a lower cost and possibly a
lower incidence of side effects than these agents (334).
There is no evidence that any single agent or any particular dose is
preferable to another. The effective doses of several progestins are summarized
722in Table 13-1. In most studies, the effect of treatment was evaluated after 3 to 6
months of therapy. Progestins appear to be an effective therapy for the painful
symptoms associated with endometriosis (335).
Medroxyprogesterone Acetate
MPA is the most studied agent. It is effective in relieving pain starting at a dose
of 30 mg per day, increasing the dose based on the clinical response and bleeding
patterns according to data from nonrandomized trials (336,337). A randomized
placebo-controlled study reported a significant reduction in stages and scores of
endometriosis in both the placebo group and the group treated with MPA 50 mg
per day and placebo at laparoscopy within 3 months after cessation of therapy
(338). These findings raise questions about the need for medical therapy with
MPA in this dose.
Evidence suggests a possible role for depot MPA in the treatment of
endometriosis. In a randomized controlled study, depot MPA (150 mg every 3
months) was more effective in the relief of dysmenorrhea than treatment with a
cyclic 21-day OC (ethinyl estradiol 20 μg plus desogestrel 0.15 mg) combined
with very low-dose danazol (50 mg per day) (339). In another multicenter,
randomized, evaluator-blinded, comparator-controlled trial, depot MPA (150 mg)
or leuprolide acetate (11.25 mg), given every 3 months for 6 months were
equivalent in reducing endometriosis-associated pain during the study and the 12-
month posttreatment follow-up period, with less impact on bone mineral density
and fewer hypoestrogenic side effects, but more bleeding being observed in the
depot MPA treated group (340). Add-back therapy would prevent the negative
effects on bone density and hypoestrogenic side effects associated with GnRHagonist therapy. In a pilot study, pain relief, side effects, and treatment
satisfaction were comparable during a 12-month treatment with etonogestrel
implantate subcutaneous (68 mg) or depot medroxyprogesterone acetate 150 mg
intramuscular depot MPA in 41 patients with dysmenorrhea, nonmenstrual pelvic
pain, and dyspareunia associated with histologically proven endometriosis (341).
Although depot MPA treatment is effective for the treatment of pain
associated with endometriosis, it is not indicated in infertile women because
it induces profound amenorrhea and anovulation, and a varying length of
time is required for ovulation to resume after discontinuation of therapy.
Dienogest
In two randomized noninferiority trials, treatment during 6 months with dienogest
2 mg per day orally demonstrated equivalent efficacy to depot leuprolide acetate
(3.75 mg, depot intramuscular injection, every 4 weeks) or intranasal buserelin
acetate (900 μg per day, intranasally) in relieving the pain associated with
723endometriosis, offering a different safety and tolerability profile (less bone loss,
fewer hot flushes, more irregular genital bleeding) (342,343). Dienogest treatment
was not compared to the recommended treatment of GnRH agonist combined
with add-back in these two trials.
Other Progestins
Megestrol acetate was administered in a dose of 40 mg per day with good results
(339). Pain was reduced significantly during luteal phase treatment with 60 mg
dydrogesterone, and this improvement was still evident at 12-month follow-up
(286). Other treatment strategies included dydrogesterone (20 to 30 mg per day,
either continuously or on days 5 to 21) and lynestrenol (10 mg per day).
Side effects of progestins include nausea, weight gain, fluid retention, and
breakthrough bleeding caused by hypoestrogenemia. Breakthrough bleeding,
although common, is usually corrected by short-term (7-day) administration of
estrogen. Depression and other mood disorders are a significant problem in about
1% of women taking these medications.
Intrauterine Progesterone
The levonorgestrel intrauterine system releasing 20 lg per day of levonorgestrel reduces endometriosis-associated pain caused by peritoneal and rectovaginal endometriosis and reduces the risk of recurrence of dysmenorrhea after conservative surgery (344). Levonorgestrel induces endometrial glandular atrophy and decidual transformation of the stroma, reduces endometrial cell proliferation, and increases apoptotic activity (344). A systematic review identified two randomized trials and three prospective observational studies, all involving small numbers and a heterogeneous group of patients (345).
The evidence suggests that the levonorgestrel intrauterine system reduces endometriosis associated pain with symptom control maintained over 3 years (346–349). Twelve months of treatment results in a significant reduction in dysmenorrhea, pelvic pain, and dyspareunia; a high degree of patient satisfaction; and a significant reduction in the volume of rectovaginal endometriotic nodules (346,347). After the first year of use, a 70% to 90% reduction in menstrual blood loss is observed.
Progesterone Antagonists and Selective Progesterone Receptor Modulators
PRAs and selective progesterone receptor modulators (SPRMs) may suppress endometriosis based on their antiproliferative effects on the endometrium, without the risk for hypoestrogenism or bone loss that occurs with GnRH treatment. Four
PRA/SPRMs have been approved by the FDA: mifepristone, ulipristal acetate (UPA), gestrinone, and asoprisnil. A Cochrane review assessed the available evidence for the use of PRAs/SPRMs in the treatment of endometriosis-associated pain (350). The authors included 10 RCTs with 960 women: two RCTs compared mifepristone versus placebo or versus a different dose of mifepristone, one RCT compared asoprisnil versus placebo, one compared ulipristal versus leuprolide
acetate, and four compared gestrinone versus danazol, gonadotropin-releasing
hormone (GnRH) analogs, or a different dose of gestrinone. The quality of
evidence ranged from high to very low. The authors conclude that there are
insufficient data about the safety and effectiveness of UPA and asoprisnil.
Mifepristone
Mifepristone is a potent antiprogestogen with a direct inhibitory effect on human endometrial cells and, in high doses, an antiglucocorticoid action (351). One study compared mifepristone with placebo and reported lower rates of dysmenorrhea (OR 0.08, 95% CI, 0.04–0.17) and dyspareunia (OR 0.23, 95% CI 0.11–0.51) in the mifepristone group (350). However, the mifepristone group had
higher rates of side effects: nearly 90% had amenorrhea and 24% had hot flushes.
Two studies compared doses of mifepristone and found insufficient evidence to
show differences between different doses in terms of effectiveness or safety, if
present. However, subgroup analysis of comparisons between mifepristone and
placebo suggest that the 2.5-mg dose may be less effective than 5 mg or 10 mg
for treating dysmenorrhea or dyspareunia.
Gestrinone
Gestrinone is a 19-nortestosterone derivative with androgenic, antiprogestogenic,
antiestrogenic, and antigonadotropic properties. It acts centrally and peripherally
to increase free testosterone and reduce sex hormone–binding globulin levels
(androgenic effect), reduce serum estradiol values to early follicular phase levels
(antiestrogenic effect), reduce mean LH levels, and obliterate the LH and folliclestimulating hormone (FSH) surge (antigonadotropic effect). Gestrinone causes
cellular inactivation and degeneration of endometriotic implants but not their
disappearance (352). Amenorrhea occurs in 50% to 100% of women and is dose
dependent.
Resumption of menses generally occurs 33 days after discontinuing the
medication (353,354). An advantage of gestrinone is its long half-life (28 hours)
when given orally. The standard dose is 2.5 mg twice a week. Although 1.25 mg
twice weekly is effective, a randomized study demonstrated in women with mild
to moderate endometriosis that 2.5 mg gestrinone twice weekly for 24 weeks is
more effective and has a better effect on bone mass (+7% vs. −7%) when
compared with 1.25 mg gestrinone twice weekly for 24 weeks (355). The clinical
side effects of gestrinone are dose dependent and similar to but less intense than those caused by danazol (355). They include nausea, muscle cramps, and
androgenic effects such as weight gain, acne, seborrhea, and oily hair and skin.
In a multicenter, randomized, double-blind study, gestrinone was as effective
as GnRH for the treatment of pelvic pain associated with endometriosis, with
fewer side effects and the added advantage of twice-weekly administration (356).
Pregnancy is contraindicated while taking gestrinone because of the risk for
masculinization of the fetus.
Danazol
Recognized pharmacologic properties of danazol include suppression of GnRH or gonadotropin secretion, direct inhibition of steroidogenesis, increased metabolic clearance of estradiol and progesterone, direct antagonistic and agonistic interaction with endometrial androgen and progesterone receptors, and immunologic attenuation of potentially adverse reproductive effects (99,357). The multiple effects of danazol produce a high-androgen, low-estrogen environment
(estrogen levels in the early follicular to postmenopausal range) that does not
support the growth of endometriosis, and the amenorrhea that is produced
prevents new seeding of implants from the uterus into the peritoneal cavity.
The immunologic effects of danazol were studied in women with
endometriosis and adenomyosis and include a decrease in serum
immunoglobulins, a decrease in serum C3, a rise in serum C4 levels, decreased
serum levels of autoantibodies against various phospholipid antigens, and
decreased serum levels of CA125 during treatment (209,358–364). Danazol
inhibits peripheral blood lymphocyte proliferation in cultures activated by T-cell
mitogens but does not affect macrophage-dependent T-lymphocyte activation of
B lymphocytes (365). Danazol inhibits IL-1 and TNF production by monocytes in
a dose-dependent manner and suppresses macrophage- and monocyte-mediated
cytotoxicity of susceptible target cells in women with mild endometriosis
(366,367). These immunologic findings may be important in the remission of
endometriosis with danazol treatment and may offer an explanation of the effect
of danazol in the treatment of a number of autoimmune diseases, including
hereditary angioedema, autoimmune hemolytic anemia, systemic lupus
erythematosus, and idiopathic thrombocytopenic purpura (368–372). Doses of
800 mg per day are used frequently in North America, whereas 600 mg per day is
prescribed in Europe and Australia. It appears that the absence of menstruation is
a better indicator of response than drug dose. A practical strategy for the use of
danazol is to start treatment with 400 mg daily (200 mg twice a day) and increase
the dose, if necessary, to achieve amenorrhea and relieve symptoms (357).
In a systematic review to determine the effectiveness of danazol compared to
placebo or no treatment for the symptoms and signs, other than infertility, of endometriosis in women of reproductive age, five randomized trials were
included in which danazol (alone or as adjunctive therapy to surgery) was
compared to placebo or no therapy (373). Treatment with danazol (including
adjunctive to surgical therapy) was effective in relieving painful symptoms
related to endometriosis when compared to placebo (373). Laparoscopic scores
improved with danazol treatment (including as adjunctive therapy) when
compared with either placebo or no treatment (373). Side effects were more
commonly reported in those patients receiving danazol than for placebo (373).
The significant adverse side effects of danazol are related to its androgenic
and hypoestrogenic properties. The most common side effects include weight
gain, fluid retention, acne, oily skin, hirsutism, hot flashes, atrophic vaginitis,
reduced breast size, reduced libido, fatigue, nausea, muscle cramps, and
emotional instability. Deepening of the voice is another potential side effect that
is nonreversible. Danazol can cause increased cholesterol and low-density
lipoprotein levels and decreased high-density lipoproteins levels, but it is unlikely
that these short-term effects are clinically important. Danazol is contraindicated in
patients with liver disease because it is largely metabolized in the liver and may
cause hepatocellular damage. Danazol is contraindicated in patients with
hypertension, congestive heart failure, or impaired renal function because it can
cause fluid retention. The use of danazol is contraindicated in pregnancy because
of its androgenic effects on the fetus.
Because of the many side effects of oral danazol, alternative routes of
administration were studied. In an uncontrolled pilot study, local danazol
treatment using a vaginal danazol ring (1,500 mg) was effective for pain relief in
deep endometriosis. This treatment did not cause the classic danazol side effects
or detectable serum danazol levels, and it allowed ovulation and conception to
occur (374).
Gonadotropin-Releasing Hormone Agonists
Gonadotropin-releasing hormone agonists bind to pituitary GnRH receptors
and stimulate LH and FSH synthesis and release. The agonists have a much
longer biologic half-life (3 to 8 hours) than endogenous GnRH (3.5 minutes),
resulting in the continuous exposure of GnRH receptors to GnRH-agonist
activity. This exposure causes a loss of pituitary receptors and downregulation of
GnRH activity, resulting in low FSH and LH levels. Ovarian steroid production is
suppressed, providing a medically induced and reversible state of
pseudomenopause. A direct effect of GnRH agonists on ectopic endometrium is
possible, because expression of the GnRH receptor gene is documented in ectopic
endometrium and because direct inhibition of endometriosis cells was shown in
vitro (375). In rat models used to study surgical adhesion formation and
727endometriosis, GnRH-agonist therapy decreased activity of plasminogen
activators and matrix MMPs and increased the activity of their inhibitors,
suggesting potential GnRH-agonist–regulated mechanisms for reducing adhesion
formation (376).
Various GnRH agonists were developed and used for treating endometriosis.
These agents include leuprolide, buserelin, nafarelin, histrelin, goserelin,
deslorelin, and triptorelin. These drugs are inactive orally and must be
administered intramuscularly, subcutaneously, or by intranasal absorption. The
best therapeutic effect is often associated with an estradiol dose of 20 to 40
pg/mL (75 to 150 pmol/L). These so-called depot formulations are attractive
because of the reduced frequency of administration and because nasal
administration can be complicated by variations in absorption rates and problems
with patient compliance (354). The results with GnRH agonists are similar to
those with OC progestin or gestrinone therapy. Treatment for 3 months with a
GnRH agonist is effective in improving pain for 6 months (295).
Although GnRH agonists do not have an adverse effect on serum lipids and
lipoproteins levels, their side effects are caused by hypoestrogenism and include
hot flashes, vaginal dryness, reduced libido, and osteoporosis (6% to 8% loss in
trabecular bone density after 6 months of therapy). Reversibility of bone loss is
equivocal and therefore of concern, because treatment periods of longer than 6
months may be required (377,378). The goal is to suppress endometriosis and
maintain serum estrogen levels of 30 to 45 pg/mL. More extreme estradiol
suppression will induce bone loss (377). The dose of daily GnRH agonist can
be regulated by monitoring estradiol levels, by the addition of low-dose
progestin or estrogen–progestin in an add-back regimen, or by draw-back
therapy.
The goal of add-back therapy is to treat endometriosis and endometriosisassociated pain effectively while preventing vasomotor symptoms and bone
loss related to the hypoestrogenic state induced by GnRH analogs. Add-back
therapy can be achieved by administering progestins only, including
norethisterone, 1.2 mg, and norethindrone acetate, 5 mg, but bone loss is not
prevented by medrogestone, 10 mg per day (378–380). Add-back therapy can be
achieved by tibolone, 2.5 mg per day, or by an estrogen–progestin combination
(i.e., conjugated estrogens, 0.625 mg, combined with medroxyprogesterone
acetate, 2.5 mg, or with norethindrone acetate, 5 mg; estradiol, 2 mg, combined
with norethisterone acetate, 1 mg) (374,377,379–383). Treatment for up to 2
years with combined estrogen and progestogen add-back appears to be effective
and safe in terms of pain relief and bone density protection; progestogen only
add-back is not protective (384).
GnRH agonists should not be prescribed to girls who have not yet attained
728their maximal bone density, as some concern remains about the long-term
effects of GnRH analogs on bone loss. In one report, bone mineral density
reduction occurred during long-term GnRH-agonist use and was not fully
recovered up to 6 years after treatment (385). Use of add-back therapy (2 mg
estradiol and 1 mg norethisterone acetate) did not affect this process (385).
Draw-back therapy was suggested as an alternative in a study showing that 6
months of intake of 400 μg per day of nafarelin was as effective as a draw-back
regimen consisting of 1 month of intake of 400 μg per day of nafarelin followed
by 5 months of 200 μg per day of nafarelin, with similar estradiol levels (30
pg/mL) but less loss of bone mineral density (386).
Aromatase Inhibitors
Treatment of rats with induced endometriosis using the nonsteroidal aromatase
inhibitor fadrozole hydrochloride or YM511 resulted in a dose-dependent volume
reduction of endometriosis transplants (387,388). In one case report, treatment of
severe postmenopausal endometriosis with an aromatase inhibitor, anastrozole, 1
mg per day, and elemental calcium, 1.5 g per day for 9 months resulted in
hypoestrogenism, pain relief after 2 months, and after 9 months a 10-fold
reduction in the 30-mm diameter size of red, polypoid vaginal lesions, along with
remodeling to gray tissue (389).
There is concern with the use of aromatase inhibitors such as anastrozole
or letrozole in the treatment of premenopausal women because these drugs
are known to stimulate ovulation and continuous administration can result in
the development of functional ovarian cysts. This side effect can be prevented
by combining aromatase inhibitors with ovarian suppressing drugs such as
OCs or progestins. A systematic review assessing the effects of aromatase
inhibitors in women symptomatic of pain with endometriosis included eight
studies including 137 women (390). In case series (seven studies, 40 women),
aromatase inhibitors combined with progestins or OCs or GnRH analogs reduced
mean pain scores and lesion size and improved quality of life (390). An RCT
including 97 women demonstrated that aromatase inhibitors in combination with
GnRH analogs significantly improved pain (p <0.0001), compared with GnRH
analogs alone, with significant improvement in multidimensional patient scores (p
<0.0001), without significant reduction in spine or hipbone densities (391).
Aromatase inhibitors appear to have a promising effect on pain associated with
endometriosis, but the strength of this inference is limited because of a dearth of
evidence and because aromatase inhibitors need to be combined with other
hormonal medication (390).
Selective Estrogen Receptor Modulators
The role of selective estrogen receptor modulators (SERMs) in the treatment of
endometriosis is unclear. In animal models, raloxifene therapy resulted in
regression of endometriosis. The effect was seen in a surgically prepared, rat
uterine explant model and in Rhesus macaques diagnosed with spontaneous
endometriosis before exposure (392). In a placebo-controlled randomized trial in
women with chronic pelvic pain and surgically treated biopsy-proven
endometriosis, postoperative treatment with raloxifene for 6 months resulted in a
shortened time to return of pain (defined as 2 months of pain equal to or more
severe than that at study entry) and to repeat laparoscopy, suggesting that
raloxifene is not effective in the treatment of endometriosis-associated pain (393).
Biopsy-proven endometriosis was not associated with return of pain, suggesting
that other factors were implicated in recurrent pelvic pain after surgery in this
study (393).
GnRH Antagonists
Gonadotrophin-releasing hormone (GnRH) antagonists inhibit the action of
endogenous GnRH through competitively and reversibly binding to GnRH
receptors in the pituitary gland (394). Unlike the GnRH agonists, which cause
an initial stimulation of the hypothalamic-pituitary-gonadal axis, GnRH
antagonists have an immediate onset of action, rapidly reducing sex hormone
levels without an initial surge. Gonadotropin-releasing hormone antagonists are
available as injectables (ganirelix, cetrorelix) and increasingly as oral nonpeptide
forms (elagolix, abarelix, ozarelix). The use of GnRH antagonists for a variety of
reproductive indications, mostly in medically assisted reproduction, has greatly
increased over the past decade.
The development of oral GnRH antagonists made these drugs potential
candidates for treatment of endometriosis. In contrast to the GnRH agonists and
injectable GnRH antagonists, that completely suppress pituitary function, oral
GnRH antagonists can produce a dose-dependent suppression of pituitary
function and production of ovarian hormones (394,395). Similar to the effect
of add-back therapy during GnRH agonist treatment, oral GnRH antagonist could
be used to suppress the estrogen production to a level that is adequate to control
endometriosis-related pain symptoms but minimizes hypoestrogenic effects.
An RCT, including 872 women in total, showed that elagolix at low (150 mg
once daily) and high dose (200 mg twice daily) was more effective than placebo
in improving dysmenorrhea and nonmenstrual pelvic pain during a 6-month
period in women with endometriosis-associated pain (395). Women who received
elagolix had higher rates of hot flushes, higher levels of serum lipids, and greater
decreases from baseline in bone mineral density than did those who received
placebo. Further studies comparing oral GnRH antagonists with other treatments are required.
Nonhormonal Medical Therapy
Progress in understanding the pathogenesis of endometriosis led to the
expectation that new pharmaceutic agents affecting inflammation and
angiogenesis activity may prevent or inhibit the development of endometriosis.
Most of these compounds were tested only in rodent models, and more research is
needed in the baboon model for endometriosis and in women to ensure their
safety and efficacy, as they may interfere with normal physiologic processes like
ovulation, menstruation, and implantation (57).
Selective Inhibition of Tumor Necrosis Factor α
In rats with experimental endometriosis, recombinant human TNF-α–binding
protein can reduce 64% of the size of endometriosis-like peritoneal lesions (396).
Several prospective randomized placebo- and drug-controlled studies in baboons
showed that TNF-α antagonists effectively prevent and treat induced
endometriosis and endometriosis-related adhesions and are effective in the
treatment of spontaneous endometriosis in baboons (57). These results were not
confirmed in a small placebo-controlled randomized trial in women with deep
endometriosis, who were awaiting surgery, possibly because the endometriosis
phenotype in these women (deep and fibrotic disease) was different from the
endometriosis phenotype in the baboon studies (inflammatory peritoneal disease
with adhesions) (397). TNF-α antagonists are less effective in fibrotic
inflammatory bowel disease than in earlier nonfibrotic inflammatory bowel
disease.
Pentoxifylline
In a systematic review determining the effectiveness and safety of pentoxifylline, which has anti-inflammatory effects in the management of endometriosis in infertile, premenopausal women, four trials involving 334 participants were included (398). Pentoxifylline had no significant effect on reduction in pain (one randomized trial, MD −1.60; 95% CI, −3.32–0.12), improvement of fertility (three randomized trials, OR 1.54; 95% CI, 0.89–2.66) or recurrence of endometriosis (one randomized trial, OR 0.88; 95% CI, 0.27–2.84) (398). No trials reported the effects of pentoxifylline on the odds of live birth rate per woman, improvement of endometriosis-related symptoms, or adverse events (398).
Peroxisome Proliferator Activated Receptor-γAgonists
Peroxisome proliferator activated receptor-γ (PPAR-γ) agonists prevent and treat endometriosis in rodent and baboon models for endometriosis and show promise for treatment of human endometriosis (399–402).
Other
Many substances potentially capable of modulating immunologic or inflammatory mechanisms involved in the onset or progression of the disease could be the targets for future research in endometriosis (403,404). Preliminary trials with cyclooxygenase-2 (COX-2) inhibitors, leukotriene receptor antagonists, TNF-α inhibitors, antiangiogenic agents and kinase inhibitors show promising results in vitro in rodent and in nonhuman primate models, but their safety is an important issue regarding human use (405).
Treatment of Endometriosis-Associated Infertility
Surgical Treatment
The treatment of endometriosis-related infertility is dependent on the age of
the woman, the duration of infertility, the stage of endometriosis, the
involvement of ovaries and tubes in the endometriosis process, previous
therapy, associated pain symptoms, and the priorities of the patient, taking
into account her attitude toward the disease, the cost of treatment, her
financial means, and the expected results. If surgery is performed and
spontaneous pregnancy does not occur within 2 years of surgery, there is little
chance of subsequent natural conception (406).
Surgery for Minimal to Mild Endometriosis
[7] Surgical management of infertile women with minimal to mild
endometriosis is controversial. Based on the results of a meta-analysis of two
randomized trials, ablation of endometriotic lesions plus adhesiolysis to
improve fertility in minimal to mild endometriosis is effective compared to
diagnostic laparoscopy alone (185,186,407). One Canadian study reported that
laparoscopic surgery enhanced fecundity in infertile women with minimal or mild
endometriosis (185). They studied 341 infertile women, 20 to 39 years of age,
with minimal or mild endometriosis. During diagnostic laparoscopy, the women
were randomly assigned to undergo resection or ablation of visible endometriosis
or diagnostic laparoscopy only. They found that resection or ablation of
minimal and mild endometriosis increased the likelihood of pregnancy in
infertile women. They were followed for 36 weeks after the laparoscopy or, for
those who became pregnant during that interval, for up to 20 weeks of pregnancy.
The study participants were recruited among infertile women scheduled for
diagnostic laparoscopy with strict eligibility criteria. The women in the study had
no previous surgical treatment for endometriosis, no medical treatment for
732endometriosis in the previous 9 months, and no other medical or surgical
treatment for infertility in the previous 3 months. They had no history of pelvic
inflammatory disease and no severe pelvic pain precluding expectant
management. The diagnosis of endometriosis required the presence of one or
more typical bluish or black lesions. The stage of endometriosis was determined
according to the revised American Society of Reproductive Medicine
classification. The MFR and the cumulative pregnancy rate after 36 weeks were
significantly higher and twice as high after surgical excision of minimal to mild
endometriosis (4.7% and 30.7%, respectively) than after diagnostic laparoscopy
(2.4% and 17.7%, respectively). In the treated group, 31% of the patients became
pregnant, compared with 18% in the nontreated group (p = 0.006). Limitations of
this study include the lack of blinding of the patients, and the fecundity rate after
surgery was below that observed in control groups from other studies (1,408).
In a multicenter study in Italy, a similar study design was used to compare the
effect of diagnostic laparoscopy with surgical resection and ablation of visible
endometriosis (on fertility parameters) in infertile women with minimal to mild
endometriosis (186). Eligible patients were less than 36 years old, were trying to
conceive, and had a laparoscopically confirmed diagnosis of minimal or mild
endometriosis. None of the women had therapy for endometriosis or infertility.
Treatment was randomly allocated during laparoscopy. There was a follow-up
period of 1 year after the laparoscopy. The results of this study did not show a
beneficial effect of surgery regarding fertility. During the follow-up period
after laparoscopy, no statistically significant differences in conception and live
birth rates were observed in the treated group (24% and 20%, respectively) or in
the control group (29% and 22%, respectively). The methodologic quality of the
Italian study was inferior to the Canadian study (175,185,186). First, the study
was underpowered, including only 91 patients, compared to 341 patients in the
Canadian study. Second, it is remarkable and unexplained that the Italian study
included after randomization more patients undergoing surgical excision of
endometriosis (n = 54) than patients undergoing diagnostic laparoscopy (n = 47).
Third, the duration of infertility was longer in the Italian study (4 years) than in
the Canadian study (32 months). Duration of infertility is an important factor
influencing MFR and cumulative pregnancy rates independently from other
causes of infertility. The bias introduced by the long duration of infertility in
couples participating in the Italian trial may have reduced the possibility to find
any significant effect of surgical treatment, especially in view of the lack of
proper power calculation in the Italian study. Fourth, the Italian study did not
present any data on MFR or cumulative pregnancy rates using life table analysis,
but only published the crude live birth rate per patient, not controlled for number
of cycles per patient. Fertility outcome should be measured by more controlled
733variables such as MFR and the cumulative pregnancy rates or time to pregnancy.
Fifth, 41 of 91 patients in the Italian study had received GnRH analog treatment
after surgery (18 from the surgical excision group, 23 from the diagnostic
laparoscopy group) (186). There was no specification for how long this medical
treatment was given and how ovarian function was affected. The lack of this
information introduces another bias influencing fertility outcome. Taking into
account the relative methodologic weaknesses of the Italian study when compared
to the Canadian study, extreme caution is needed before combining these two
studies in a meta-analysis, especially because the fertility outcome data are
reported so differently. It seems preferable to use the data of the better study
demonstrating that surgical treatment of minimal to mild endometriosis
appears to offer a small, but significant, benefit with regard to fertility
outcome (1,175,185,409). The surgical removal of peritoneal endometriosis may
be important to prevent progression of endometriosis. Care is needed to prevent
adhesion formation that could result as a consequence of overenthusiastic
excision of minimal to mild endometriosis.
Surgery for Moderate to Severe Endometriosis
When endometriosis causes mechanical distortion of the pelvis, surgery should be
performed to achieve reconstruction of normal pelvic anatomy. No randomized
trials or meta-analyses are available to answer the question of whether surgical
excision of moderate to severe endometriosis enhances pregnancy rate (1). Most
studies present only crude pregnancy rates without detailed information regarding
time of follow-up and are therefore not relevant (1).
Based on three studies, there seems to be a negative correlation between the
stage of endometriosis and the spontaneous cumulative pregnancy rate after
surgical removal of endometriosis, but statistical significance was reached in only
one study (410–412).
Other studies reported a significant negative correlation between endometriosis
stage and pregnancy rate and decreased pregnancy rates when the revised scores
exceeded 15 or 70 (177,410,413). Data from different studies cannot be compared
easily because of retrospective design, lack of a control group, significant
variability and lack of standardization with respect to inclusion criteria, surgical
procedures, extent of surgery, skill of the surgeon, variable duration of follow-up
without life table analysis, postoperative hormonal suppression or medically
assisted reproduction, and lack of control for other infertility-related factors such
as male infertility or ovarian dysfunction. These limitations explain why
management differed and was not standardized, and why the cumulative
pregnancy rates 9 to 12 months after surgery for moderate to severe endometriosis
vary between 24% and 30% (1,414–416).
734Surgery for Ovarian Endometriomas in Infertile Patients
In patients with an endometrioma receiving surgery for infertility or pain,
cystectomy of the endometrioma increased the postoperative spontaneous
pregnancy rate, compared to ablation of the endometrioma (417). Therefore, the
ESHRE guideline recommends cystectomy over ablation of endometriomas
in infertile women with an ovarian endometrioma undergoing surgery (1).
A randomized study demonstrated an increased ovarian follicular
response to gonadotrophin stimulation for women who underwent excisional
surgery when compared to ablative surgery (weighted mean difference
[WMD] 0.6; 95% CI, 0.04–1.16) (418). There is insufficient evidence to favor
excisional surgery over ablative surgery with respect to the chance of pregnancy
after controlled ovarian stimulation and intrauterine insemination (OR 1.40; 95%
CI, 0.47–4.15) or treatment with assisted reproductive technology (272).
Cystectomy and ablation of an endometrioma carry potential risks for the
ovarian reserve, either by removal of normal ovarian tissue during excision
or by thermal damage to the ovarian cortex during ablation. Although as little
as one-tenth of an ovary may be enough to preserve function and fertility, there is
concern that ovarian cystectomy with concomitant removal or destruction of
normal ovarian tissue may reduce ovarian follicle reserve and reduce fertility
(419). In a small prospective randomized clinical trial, 10 patients assigned to
undergo laparoscopic cystectomy had a lower antral follicle count and a more
pronounced reduction in serum anti-müllerian hormone (AMH) levels (from 3.9
to 2.9 ng/mL) 6 months after surgery when compared to the 10 patients treated
with a “three-stage procedure” as described above (reduction in AMH levels from
4.5 to 3.99 ng/mL) (420,421). More randomized trials are needed to assess the
effect of ovarian cystectomy on ovarian reserve and on reproductive function,
especially with respect to conception after treatment with medically assisted
reproduction.
Preoperative and Postoperative Medical Treatment
As described in the section on conservative surgery for endometriosis-associated
pain, there is no evidence of a benefit of preoperative medical therapy
(danazol, progestins, GnRH agonists) on the outcome of endometriosis
surgery. However, in patients with severe endometriosis, it is common clinical
practice that surgical treatment be preceded by a 3-month course of medical
treatment because it is believed that this facilitates surgery by reducing
inflammation and vascularization of lesions.
Postoperative medical treatment is not indicated because it is ineffective
based on randomized trials, prevents pregnancy, and because the highest
spontaneous pregnancy rates occur during the first 6 to 12 months after
735conservative surgery (296,298).
Hormonal Treatment
Conception is either impossible or contraindicated during medical treatment
of endometriosis. [8] There is no evidence that medical treatment of minimal
to mild endometriosis leads to better chances of pregnancy than expectant
management (408). The published evidence does not comment on more
severe disease (1).
Medically Assisted Reproduction
Medically assisted reproduction—including controlled ovarian hyperstimulation
with intrauterine insemination, IVF, and gamete intrafallopian transfer—may be
an option for infertility treatment in addition to surgical reconstruction and
expectant management (266). Assisted reproductive technology (ART) is the
method of choice when distortion of the tubo-ovarian anatomy contraindicates the
use of superovulation with intrauterine insemination or gamete intrafallopian
transfer (266).
The role of ART in the treatment of endometriosis-associated infertility may be
limited in large tertiary care and referral centers for surgical treatment of
endometriosis (422). After conservative surgery for endometriosis, 44%
conceived in vivo (44%), and 51% of those who failed to conceive in vivo did not
undergo ART treatment with the cumulative rate of IVF use at 36 months of
infertility at 33%. The live birth/ongoing pregnancy rate per started cycle and per
patient was 10% and 20%, respectively (422). For a full discussion on the
application of ART in infertility, see Chapter 36.
Management of Adolescents
The most common presenting symptom in adolescents with endometriosis is
cyclic pain (1). Less commonly acyclic pain, dyspareunia, gastrointestinal
symptoms, irregular menses, urinary symptoms, and vaginal discharge are
described (1,423–426). Similar presenting symptoms occur in adolescent patients
evaluated for pelvic pain with and without endometriosis (426,427). It is hard to
predict the presence of endometriosis in adolescents with pelvic pain merely
from the presenting symptoms, because similar symptoms occur in patients
evaluated laparoscopically for pelvic pain with and without endometriosis
(1,426,427). Laparoscopy should be considered for adolescents with chronic
pelvic pain who do not respond to medical treatment (NSAIDs, OCs) because
endometriosis is very common (up to 70%) under these circumstances (423,426–
433). Minimal and mild endometriosis, according to the revised ASRM
736classification, are the most common stages of the disease in adolescents.
Gynecologic surgeons should pay special attention to red, clear, or white lesions,
which are more prevalent in adolescents than in adults with endometriosis (1,423–
435). Mild disease can be treated by laparoscopic surgical removal of implants at
the time of diagnosis, followed by continuous administration of low-dose
combination OCs to prevent recurrence. More advanced disease can be treated
medically for 6 months, followed by continuous OCs to prevent progression of
disease. Surgery is indicated if this hormonal treatment is not effective. GnRH
agonists with add-back therapy can be considered for only adolescents older
than 17 years of age who have completed pubertal and bone maturation, and
then only if symptoms persist during other forms of hormonal suppression
(1,436–438).
Menstrual outflow obstructions such as müllerian anomalies may cause
early development of endometriosis in adolescents. Regression of the disease
was observed when surgical correction of the anomaly was accomplished (439–
441).
Evidence suggests that absenteeism from school and the incidence and
duration of OC use for severe primary dysmenorrhea during adolescence is
higher in women who later develop deep endometriosis than in women
without deep endometriosis (160).
Physicians treating adolescents with endometriosis should adopt a
multidimensional approach, where surgery, hormonal manipulation, pain
medication, mental health support, complementary and alternative therapies, and
education in self-management strategies are useful components (1).
Management of Postmenopausal Women
Estrogen dependence is a central pathophysiologic hallmark of endometriosis,
supported by a substantial body of molecular studies and clinical observations of
symptom regression in hypoestrogenic states. Therefore, endometriosis is
typically regarded as a disease of the reproductive years, resolving after
natural or iatrogenic menopause. There is the concern that hormone
replacement therapy (HRT) may reactivate endometriotic lesions, induce
development of new lesions, or stimulate malignant transformation. However, a
systematic review identified only 17 cases of recurrent endometriosis and 25
cases of malignant transformation in postmenopausal women taking some form of
HRT (442). Only one RCT evaluated the potential risk of HRT in women with
iatrogenic (bilateral oophorectomy) menopause. In a small RCT, recurrence of
pain was not significantly different in any of 57 patients in the no-treatment arm,
or in 4 of 115 women receiving sequential administration of estrogens and
progesterone with two 22-cm patches applied weekly to produce a controlled
737release of 0.05 mg per day, combined with oral administration of micronized
progesterone administered orally (200 mg per day) for 14 days with a 16-day
interval free of treatment (6,443). In this study, the endometriosis recurrence and
reoperation rate were comparable in both groups (2 of 115 of the treatment group;
0 of 57of the no-treatment group) (443). Two observational studies support the
findings of this RCT. The first study included 107 women with (n = 90) or
without (n = 17) HRT after iatrogenic (hysterectomy and BSO) menopause (444).
Recurrence was only identified in four women, 3 women had recurrent pain and
one woman had a vaginal nodule. All patients with recurrence were receiving
unopposed estrogen therapy. The second observational study reported on 42
women with deep infiltrating endometriosis (445). Nineteen of these 42 patients
had a hysterectomy and BSO and 11 were subsequently treated with HRT. During
the follow up period (mean = 4.3 years, range 1 to 18) no women were diagnosed
with recurrence of endometriosis.
This systematic review shows that postmenopausal recurrence or malignant
transformation of endometriosis is possible but very rare. The authors
conclude that HRT in patients with a history of endometriosis has potential risks,
but these risks are likely very small and the substantial benefits of HRT should
not be overlooked, especially in women with iatrogenic menopause at a young
age. This is in line with the ESHRE guideline, which states that women should
not be denied HRT treatment simply because of a history of endometriosis.
Based on the very limited available data, unopposed estrogen treatment in
postmenopausal hysterectomized women with a history of endometriosis is
dissuaded. In young women with endometriosis undergoing iatrogenic
(surgical) menopause the ESHRE guideline recommends HRT with
combined estrogen/progestogen or tibolone, at least up to the age of natural
menopause (1).
Management of Recurrent Endometriosis
Recurrence After Medical Treatment
Because hormonal suppressive treatment does not cure endometriosis (it only
suppresses the activity of endometriotic lesions during the treatment),
“recurrence,” or rather persistence, of endometriosis can be expected in nearly all
patients within 6 months to 2 years after the cessation of medical treatment, and
this is positively correlated with the severity of endometriosis.
Recurrence After Conservative Surgery
Conservative surgery for endometriosis is associated with high recurrence
rates. Data regarding recurrence rates after conservative surgical treatment of
738endometriosis are scarce and systematic reviews identified considerable variation
between different studies in the reporting of postoperative recurrence rates of
endometriosis or endometriosis-associated symptoms, mostly because of the lack
of clear definitions for recurrence. A consensus on precise definitions for
suspected recurrence, based on symptoms or imaging, and proven recurrence of
endometriosis, based on laparoscopic visualization or histology, was published.
The overall endometriosis recurrence rate is estimated between 2% to
20% per year. Cumulative recurrence rates vary from 4% to 25% at 2 years
follow-up to 40% after 5 years (279,446). Recurrence of endometriosis can be the
result of incomplete surgery or caused by the development of new lesions. The
ASRM classification system has low value to predict pain recurrence and
endometriosis relapse after conservative surgical treatment (447).
Recurrence After Hysterectomy
The medium-term outcome of hysterectomy for endometriosis-associated pain is
satisfactory; the probability of pain persistence after hysterectomy is 15% and risk
of pain worsening is 3% to 5%, with a six times higher risk of further surgery in
patients with ovarian preservation as compared to concomitant bilateral ovarian
removal (448). At least one ovary should be preserved in young women,
especially in those who cannot or will not receive estrogen–progestin therapy
(449). The risk of recurrence of endometriosis during hormonal therapy seems
marginal if combined preparations or tibolone are used and estrogen-only
treatments are avoided (449).
Risk Factors for Recurrence
The rate of recurrence increases with the stage of disease, the duration of
follow-up, and the occurrence of previous surgery (15,450–453). The
likelihood of recurrence appears to be lower when endometriosis is located only
on the right side of the pelvis rather than when the left side is involved (453). The
risk of endometriosis recurrence is significantly correlated to the age of the
patient. The younger the patient is at diagnosis the higher the risk of recurrence.
Prevention of Recurrence
After first-line surgery for endometriosis, women should be invited to seek
conception as soon as possible. Alternatively, OC use until pregnancy is
desired should be considered because several lines of evidence suggest that
ovulation inhibition reduces the risk of endometriosis recurrence (454). A
recurrent endometrioma developed in 26 of 250 regular users (10%; 95% CI, 7–
15) compared with 46 of 115 never users (40%; 95% CI, 31–50), with a common
OR of 0.16 (95% CI, 0.04–0.65) (454).
739Medical Treatment of Recurrence
It has been shown that medical treatments may provide a good efficacy for
the treatment of pain in women presenting with recurrent endometriosis
(455). In a randomized prospective clinical study, continuous treatment for 6
months with desogestrel (75 μg per day) (n = 20) versus a combined OC (ethinyl
estradiol 20 μg plus desogestrel 150 μg) resulted in a significant and comparable
improvement of pelvic pain and dysmenorrhea with breakthrough bleeding in
20% of the desogestrel-treated patients, and a significant body weight increase in
15% of the OC-treated women (455).
Surgical Treatment of Recurrence
The optimal surgical solution in women with recurrent symptoms after previous
conservative procedures for endometriosis should be based on the desire for
conception and on psychological characteristics (456). Repeat conservative
surgery for DE has the same efficacy and limitations as primary surgery. The
long-term probability of pain recurrence after repeat conservative surgery
for recurrent endometriosis varies from 20% to 40%, and a further surgical
procedure will occur in 15% to 20% of the cases (448,456). These figures are
probably an underestimate related to drawbacks in study design, exclusions of
dropouts, and publication bias and should be considered with caution (456). No
studies evaluated the association of PSN to the surgical treatment of recurrent
endometriosis among patients with recurrent disease (448). When a second
surgical approach is intended, definitive surgery (hysterectomy and bilateral
oophorectomy) should be considered, particularly in women over 40 years
old and who do not wish to conceive. The spontaneous conception rate among
women undergoing repetitive surgery for recurrent endometriosis associated with
infertility is 20% (12- and 24-month cumulative pregnancy rates of 14% and
26%), whereas the overall crude pregnancy rate after a primary surgical procedure
is 40% (12- and 24-month cumulative pregnancy rates of 32% and 38%)
(448,456). Among infertile patients treated with repetitive surgery for recurrent
endometriosis the spontaneous pregnancy rate was 19% (12- and 24-month
cumulative pregnancy rates), whereas it was 34% for those untreated (12- and 24-
month cumulative pregnancy rates of 25% and 30%). The probability of
conception after IVF is not significantly lower after repetitive surgery (20%)
when compared to primary surgery (30%) (hazard ratio [HR] 1.51; 95% CI, 0.58–
3.91) (456).
740COPING WITH DISEASE
Coping with endometriosis as a chronic disease is an important component of
management. Patient self-help groups can provide invaluable counseling,
support, and advice. ESHRE provides a comprehensive international list of selfhelp groups on their web site (1). Many women with endometriosis report that
nutritional and complementary therapies such as reflexology, traditional Chinese
medicine, herbal treatments, and homeopathy improve pain symptoms. Data on
the effectiveness of alternative and complementary therapies for endometriosisassociated pain are scarce. The limited available evidence indicates that the
effectiveness of transcutaneous electrical nerve stimulation (TENS), dietary
supplements, acupuncture, and traditional Chinese medicine is not well
established for treatment of endometriosis-associated pain. Hence ESHRE does
not recommend the use of alternative and complementary therapies in the
management of endometriosis. Although there is no good evidence to support the
effectiveness of these treatments in endometriosis, they should not be ruled out if
the woman feels they work in conjunction with more traditional therapies or that
they could be beneficial to her overall pain management and quality of life

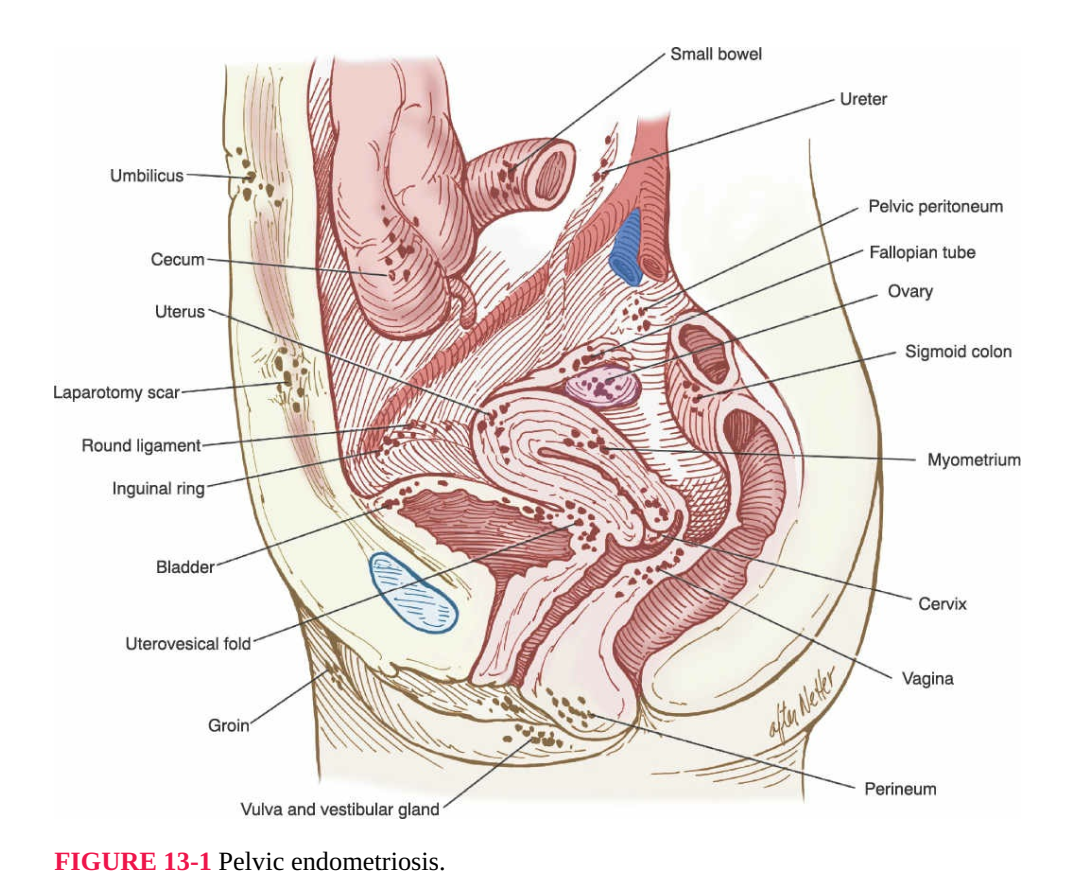










Nhận xét
Đăng nhận xét