Vulvar Cancer
BS. Nguyen Hong Anh
KEY POINTS
1 Vulvar lesions require biopsy to avoid delay in diagnosis.
2 The modern approach to patients with vulvar cancer is multidisciplinary and
individualized.
3 Management of the primary lesion and groin nodes is determined separately.
4 Most T1 and early T2 lesions can be managed with radical local excision.
5 Large T2 and T3 primary tumors are best treated with chemoradiation followed by
limited surgical resection.
6 Unifocal tumors less than 4 cm in diameter with clinically negative nodes are suitable
for sentinel lymph node dissection in experienced hands.
7 When groin dissection is indicated, it should be a thorough inguinofemoral
lymphadenectomy.
8 The single most important prognostic factor is lymph node status: 5-year survival
without groin node metastases is greater than 90%; with groin node metastases, 5-
year survival is 50%.
9 Postoperative (chemo-) radiation decreases the risk of groin recurrence in patients
with multiple positive inguinofemoral lymph nodes.
10 Recurrence in the groin is almost universally fatal.
With 6,020 new cases and 1,150 deaths annually in the United States, vulvar
cancer is uncommon, resulting in age-adjusted incidence rates of 2.8 and 1.7 per
100,000 in white and black women, respectively. Vulvar cancer represents about
4% to 6% of malignancies of the female genital tract and 0.6% of all cancers in
women (1,2). Vulvar cancer predominantly affects postmenopausal women, and it
is the most common anogenital cancer in women more than 70 years of age (3).
HPV infection is associated with a significant number of vulvar cancers. [1] The
incidence of in situ vulvar cancer has been increasing worldwide, primarily
because of the increasing occurrence in young women, who account for 75%
of the cases. The incidence of lichen sclerosus, which is associated with an
increased risk of vulvar cancer, has doubled over the past two decades,
predominantly in postmenopausal women (4). The overall rate of invasive
vulvar carcinoma is increasing, but at a much lower rate (5,6). Squamous cell
carcinomas account for over 80% of all primary vulvar malignancies, whereas
melanomas, adenocarcinomas, basal cell carcinomas, Paget disease, and sarcomas
2695are less common. Most vulvar cancers are diagnosed at an early stage. In the
United States, 5-year survival rates for vulvar cancer are 72%.
Following the initial reports of Taussig in the United States and Way in Great
Britain, radical vulvectomy and en bloc groin dissection, with or without pelvic
lymphadenectomy, was standard treatment for all patients with operable disease
(7,8). Postoperative morbidity was high and prolonged hospitalization common.
Over the past 4 decades, significant advances in the management of vulvar
cancer have been made, reflecting a paradigm shift toward a more
conservative surgical approach without compromised survival and with
markedly decreased physical and psychological morbidity:
1. [2] Individualization of treatment for all patients with invasive disease.
2. Vulvar conservation for patients with unifocal tumors and an otherwise
normal vulva.
3. Omission of the groin dissection for patients with microinvasive tumors
(T1a, Ä2 cm diameter and Ä1 mm of stromal invasion).
4. Elimination of routine pelvic lymphadenectomy.
5. Incorporation of the sentinel lymph node procedure to eliminate complete
inguinofemoral lymphadenectomy in node-negative patients who may not
derive benefits from the procedure.
6. The use of separate incisions for the groin dissection to improve wound
healing.
7. Omission of the contralateral groin dissection in patients with lateral T1
lesions and negative ipsilateral nodes.
8. The use of preoperative radiation therapy to obviate the need for
exenteration in patients with advanced disease.
9. The use of postoperative (chemo-) radiation therapy to decrease the
incidence of groin recurrence in patients with multiple positive groin
nodes.
ETIOLOGY
The etiology of vulvar cancer is multifactorial. Reported risk factors for vulvar
cancer include human papillomavirus (HPV) infection, vulvar intraepithelial
neoplasia (VIN), cervical intraepithelial neoplasia (CIN), lichen sclerosus,
cigarette smoking, alcohol consumption, obesity, immunosuppression, a prior
history of cervical cancer, and northern European ancestry (9,10). Growing
evidence has established two distinct etiologic entities of squamous cell
carcinoma of the vulva:
26961. Basaloid or warty types, which comprise about 40% of vulvar squamous
cell carcinomas, tend to be multifocal, occur generally in younger
patients, and are related to HPV infection, usual type VIN (uVIN),
immunosuppression, and cigarette smoking.
2. Keratinizing, differentiated, or simplex types, which account for about 60%
of cases, tend to be unifocal, occur predominantly in older patients, are
not related to HPV, and often are found in areas adjacent to lichen
sclerosus, chronic inflammatory dermatoses, and differentiated type VIN
(dVIN).
High-grade VIN was closely studied as a potential precancerous lesion. The
direct progression of VIN to cancer is difficult to document, but a review of 3,322
published patients with high-grade VIN reports a 9% progression rate to cancer
for untreated and 3.8% for treated cases (11). VIN is found adjacent to basaloid or
warty vulvar squamous cell carcinomas in more than 80% of cases and 10% to
20% of vulvar carcinoma in situ lesions harbor an occult invasive component
(12,13). A meta-analysis comprising 5,015 vulvar cancers demonstrated a 40%
prevalence of HPV in vulvar cancer with nearly 80% of warty or basaloid cancers
testing positive for HPV, compared with only 13% of keratinizing carcinomas.
The predominant high-risk HPV type was HPV16, followed by HPV33 and
HPV18 (14). Epidemiologic risk factors for the basaloid or warty-type
squamous cell carcinoma of the vulva are similar to those for cervical cancer
and include a history of multiple lower genital tract neoplasias,
immunosuppression, and smoking. Based on immunogenicity and efficacy
studies, it is expected that HPV vaccination, particularly with the nine-valent
HPV vaccine, has the potential to prevent up to 90% of HPV-associated vulvar
cancers (15).
Frequently implied as an etiologic variable for the keratinizing carcinoma is the
itch–scratch cycle associated with lichen sclerosus and chronic inflammatory
dermatoses, with atypical changes occurring in the repaired epithelium.
Differentiated VIN is a precursor to keratinizing squamous cell carcinoma and is
strongly associated with lichen sclerosus. In keratinizing carcinoma, adjacent
lichen sclerosus or dVIN is found in more than 80% of patients (4). Women with
vulvar lichen sclerosus are at increased risk of developing invasive squamous
cell cancer of the vulva. A population-based study found a 36-fold increased risk
of vulvar cancer for women with lichen sclerosus (16). During the past 20 years,
the incidence of lichen sclerosus has nearly doubled from 7.4 to 14.6 per 100,000
woman-years. In a cohort of more than 3,000 women with lichen sclerosus, the
observed cumulative incidence of squamous cell carcinoma of the vulva was
6.7% with concurrent VIN and age ≥70 years being independent risk factors (4).
2697Supportive evidence that some of these lesions are precancerous comes from
molecular studies that demonstrate aneuploid DNA content, p53 overexpression,
high Ki67 expression, indicating high proliferation indices and monoclonal
expansion of keratinocytes in lichen sclerosus and dVIN (17–19), though the
exact molecular mechanism of non–HPV-associated vulvar cancer remains to be
elucidated. An area of active research explores whether treatment of lichen
sclerosus with superpotent topical steroids can impact the malignancy risk (20). A
prospective longitudinal cohort study of 507 women with biopsy-proven vulvar
lichen sclerosus demonstrated that patients who were compliant with active
treatment, typically with superpotent topical steroids, had improved symptom
control and a reduced incidence of vulvar carcinoma (21).
TYPES OF INVASIVE VULVAR CANCER
The histologic subtypes of invasive vulvar cancer are shown in Table 40-1.
Squamous Cell Carcinoma
Approximately 90% to 92% of all invasive vulvar cancers are of the
squamous cell type. Squamous carcinomas of the vulva can be divided into
distinct histologic subtypes designated as basaloid carcinoma, warty carcinoma,
and keratinizing squamous carcinoma. Mitoses are noted in these malignancies,
but atypical keratinization is the histologic hallmark of invasive vulvar cancer
(23). Most vulvar squamous carcinomas reveal keratinization (Fig. 40-1).
Histologic features that correlate with the occurrence of inguinal lymph node
metastasis are lymph–vascular space invasion, tumor thickness, depth of stromal
invasion, grade of differentiation, histologic pattern of invasion (spray and stellate
versus confluent, compact, broad, and pushing), and increased amount of keratin
(24–27).
Table 40-1 Malignant Tumors of the Vulva Based on IARC/WHO Classification (22)
Type Percent
Malignant Epithelial Tumors
Squamous cell carcinoma (keratinizing, non-keratinizing, basaloid,
warty, verrucous)
90–
92%
Basal cell carcinoma 2–
3%
Paget disease <1%
2698Bartholin gland (adenocarcinoma, squamous cell, adenosquamous,
adenoid cystic, transitional cell)
1%
Tumors arising from other specialized anogenital glands
(adenocarcinoma of mammary gland type, adenocarcinoma of Skene
gland or minor vestibular gland origin, malignant Phylloides tumor)
<1%
Adenocarcinomas of other types (sweat gland type, intestinal type) <1%
Malignant Neuroectodermal Tumors <1%
Ewing sarcoma
Malignant Soft Tissue Tumors <1%
Rhabdomyosarcoma
Leiomyosarcoma
Epithelioid sarcoma
Alveolar soft part sarcoma
Other sarcomas
Malignant Melanocytic Tumors
Malignant melanoma 2–
5%
Malignant Germ Cell Tumors <1%
Neuroendocrine tumors (small cell or large cell high-grade
neuroendocrine carcinoma)
Merkel cell tumor
Yolk sac tumor
Lymphoid and Myeloid Tumors <1%
Lymphomas
Myeloid neoplasms
Secondary (metastatic) Tumors 1%
2699IARC, International Agency for Research on Cancer; WHO, World Health Organization.
[3] Microinvasive carcinoma of the vulva (T1a) is defined as a lesion 2 cm
or less in diameter with 1 mm or less stromal invasion (28). Depth of stromal
invasion is measured vertically from the epithelial–stromal junction (basement
membrane) of the adjacent most superficial dermal papilla to the deepest point of
tumor invasion (Fig. 40-2). When the tumor invades 1 mm or less, metastasis to
the inguinal lymph nodes is extremely rare among reported series. When
invasion is greater than 1 mm, there is a significant risk of inguinal lymph
node metastasis.
Clinical Features
[4] Squamous cell carcinoma of the vulva is predominantly a disease of
postmenopausal women. The mean age at diagnosis is about 65 years and
15% of patients who develop vulvar cancer do so before age 40. There may
be a longstanding history of an associated vulvar intraepithelial disorder,
such as lichen sclerosus or VIN. As many as 27% of patients with vulvar
cancer have a second primary malignancy (29–31). Based on data from the
National Cancer Institute’s Surveillance Epidemiology and End Results (SEER)
program, patients with invasive vulvar cancer have an increased risk of 1.3% for
developing a subsequent cancer. Most of the excess second cancers were
smoking related (e.g., cancers of the lung, buccal cavity and pharynx, esophagus,
nasal cavity, and larynx) or related to infection with HPV (e.g., cervix, vulva,
vagina, and anus) (32).
Most patients are asymptomatic at the time of diagnosis. If symptoms exist,
vulvar pruritus, a lump, or a mass are the most common findings. Less frequent
symptoms include a bleeding or ulcerative lesion, discharge, pain, or dysuria.
Occasionally, a large metastatic mass in the groin is the initial symptom.
A careful inspection of the vulva should be part of every gynecologic
examination. On physical examination, vulvar carcinoma is usually raised and
may be fleshy, ulcerated, plaquelike, or warty in appearance. It may be
pigmented, red or white, and tender or painless. The lesion may be clinically
indistinct, especially in the presence of VIN, lichen sclerosus, or other vulvar
dermatoses (13). Any lesion of the vulva warrants a biopsy.
FIGURE 40-1 Squamous cell carcinoma of the vulva, keratinizing type. The multiple
pearl formations consist of laminated keratin.
FIGURE 40-2 Early invasive carcinoma of vulva originating from vulvar intraepithelial
neoplasia. An irregular nest of malignant cells extend from the base of rete pegs.
Desmoplastic stromal reaction and chronic inflammation are useful diagnostic signs of
stromal invasion. The depth of stromal invasion is measured from the base of the most
superficial dermal papilla vertically to the deepest tumor cells.
Most squamous carcinomas of the vulva occur on the labia majora and
minora (60%), but the clitoris (15%) and perineum (10%) may be primary
sites. Approximately 10% of the cases are too extensive to determine a site of
origin, and about 5% of the cases are multifocal.
As part of the clinical evaluation, a careful assessment of the extent of the
lesion, including whether it is unifocal or multifocal, should be performed. The
lesion diameter should be measured and its proximity to the midline and/or
involvement of midline structures including urethra, vagina, clitoris, and anus
should be assessed. The groin lymph nodes should be evaluated carefully, and a
complete pelvic examination should be performed. A cytologic sample should be
taken from the cervix, and colposcopy of the vulva, cervix, and vagina should
be performed because of the common association with other squamous
2702intraepithelial or invasive neoplasms of the lower genital tract.
Diagnosis
Diagnosis requires a Keys punch biopsy or wedge biopsy, which can be
obtained in the office using local anesthesia. The biopsy must include sufficient
underlying dermis to assess for microinvasion.
Physician delay is a common problem in the diagnosis of vulvar cancer,
particularly if the lesion has a warty appearance. Any large or confluent
warty lesion requires biopsy before medical or ablative therapy is initiated.
Routes of Spread
Vulvar cancer spreads by the following routes:
1. Direct extension, to involve adjacent structures such as the vagina, urethra,
clitoris, and anus.
2. Lymphatic embolization to the regional inguinal and femoral lymph nodes.
3. Hematogenous spread to distant sites, including the lungs, liver, and bone.
[5] Lymphatic metastases may occur early in the disease. Twelve percent of
tumors 2 cm in diameter or smaller have regional metastases (29,33).
Initially, spread is usually to the inguinal lymph nodes, which are located between
Camper’s fascia and the fascia lata (34). From these superficial groin nodes, the
disease spreads to the deep femoral nodes, which are located medial to the
femoral vessels (Fig. 40-3). Cloquet’s or Rosenmüller’s node, situated beneath the
inguinal ligament, is the most cephalad of the femoral node group. Metastases to the
femoral nodes without involvement of the inguinal nodes are reported (35–
38). A study from the M. D. Anderson Cancer Center reported a 9% groin
recurrence rate in 104 patients with vulvar cancer who had negative nodes on
superficial inguinal lymphadenectomy at initial surgery, and intraoperative
lymphatic mapping studies found the sentinel node deep to the cribriform fascia
in 5% to 16% of these cases (39–41). This pattern of lymphatic spread needs to be
taken into consideration when determining the extent of inguinofemoral
lymphadenectomy and the radiotherapeutic techniques and target volumes.
From the inguinal–femoral nodes, the cancer spreads to the pelvic nodes,
particularly the external iliac group. Although direct lymphatic pathways from
the clitoris and Bartholin gland to the pelvic nodes were described, these channels
seem to be of minimal clinical significance (42–44). The lymphatics from either
side of the vulva form a rich network of anastomoses along the midline.
Lymphatic drainage from the clitoris, anterior labia minora, and perineum is
bilateral. Metastases to contralateral lymph nodes in the absence of ipsilateral
2703nodal involvement is very rare (0% to 0.4%) for lateral vulvar tumors that are
either 2 cm or less in diameter or with 5 mm or less invasion (33,45).
The overall incidence of inguinal–femoral lymph node metastases is
reported to be about 32% (38,40,43–53) (Table 40-2). Metastases to pelvic
nodes occur in about 12% of cases. Pelvic nodal metastases are rare (0.6%) in
the absence of groin node involvement, but they occur in about 16% of cases with
positive groin nodes (Table 40-2). The risk increases to 33% in the presence of
clinically suspicious groin nodes and to 40% to 50% if there are three or more
pathologically positive inguinal–femoral nodes (30,45,54–57). The incidence of
lymph node metastases correlates positively with depth of invasion, as shown in
Table 40-3. Hematogenous spread usually occurs late in the course of vulvar
cancer and is rare in the absence of lymph node metastases.
FIGURE 40-3 Inguinal–femoral lymph nodes. (From Hacker NF. Vulvar and Vaginal
Cancer. In: Hacker NF, Gambone J, Hobel C. Hacker & Moore’s Essentials of Obstetrics
and Gynecology. 6th ed. Philadelphia, PA: Elsevier Saunders; 2015:452, with permission.)
Table 40-2 Incidence of Lymph Node Metastases in Squamous Cell Carcinoma of the
Vulva
2704Staging
Initially, vulvar carcinoma was staged clinically based on tumor size and location,
palpable regional lymph node status, and a limited search for distant metastases.
The prognostic importance of the lymph node status is significant, but clinical
assessment of the lymph nodes has limited accuracy. This led the Cancer
Committee of FIGO to introduce a surgical staging system for vulvar cancer in
1988, which underwent several revisions, most recently in 2009, to provide better
prognostic discrimination between stages and less heterogeneity within stages
(28,58,59) (Table 40-4), by separating patients whose tumors involve adjacent
perineal structures from those with positive lymph nodes and taking into account
the number and morphology of the involved nodes.
The new FIGO staging system was validated in 269 patients, 42% of whom
were restaged (61). The number of positive nodes negatively correlated with
survival, as did the presence of extracapsular growth. This study confirmed
Gynecologic Oncology Group (GOG) and SEER data indicating that in patients
with negative nodes, tumor size was not predictive of survival (59). This study
confirmed reports demonstrating that, when corrected for the number of positive
nodes, bilaterality of nodal metastases was not predictive of survival (61–65).
Paralleling the changes to the FIGO staging, the American Joint Committee on
Cancer (AJCC) significantly modified the vulvar cancer tumor-node-metastasis
(TNM) classification with the release of the 2009 edition of the AJCC Cancer
Staging Manual (Table 40-4) (60).
2705Table 40-3 Nodal Status in T1 Squamous Cell Carcinoma of the Vulva Versus Depth
of Stromal Invasion
Depth of Invasion No. of Patients Positive Nodes Nodes
1 mm 163 0 0
1.1–2 mm 145 11 7.7
2.1–3 mm 131 11 8.3
3.1–5 mm 101 27 26.7
>5 38 13 34.2
Total 578 62 10.7
From Hacker NF, Eifel PJ. Vulvar cancer. In: Berek JS, Hacker NF. Berek & Hacker’s
Gynecologic Oncology. 6th ed. Philadelphia, PA: Wolters Kluwer; 2015:569.
Table 40-4 The 2009 FIGO Staging and TNM Classification for Vulvar Cancer
(28,61)
FIGO Stage TNM Classification Clinical/Pathologic Findings
Stage IA T1aN0M0 Lesions ≤2 cm in size, confined
to the vulva or perineum and with
stromal invasion ≤1 mm,a no
nodal metastasis
Stage IB T1bN0M0 Lesions >2 cm in size or with
stromal invasion >1 mm,
confined to the vulva or
perineum, with negative nodes
Stage II T2N0M0 Tumor of any size with extension
to adjacent perineal structures
(1/3 lower urethra, 1/3 lower
vagina, anus) with negative nodes
Stage III Tumor of any size with or without
extension to adjacent perineal
structures (1/3 lower urethra, 1/3
lower vagina, anus) with positive
inguinofemoral lymph nodes
2706IIIA T1or2 N1b M0 T1or2 N1a
M0
(i) with 1 lymph node metastasis
(≥5 mm) or (ii) 1–2 lymph node
metastasis(es) (<5 mm)
IIIB T1or2 N2b M0 T1or2 N2a
M0
(i) with 2 or more lymph node
metastasis (≥5 mm) or (ii) 3 or
more lymph node metastases (<5
mm)
IIIC T1or2 N2c M0 with positive nodes with
extracapsular spread
Stage IV Tumor invades other regional (2/3
upper urethra, 2/3 upper vagina),
or distant structures
IVA T3Nany M0 TanyN3 Tumor invades any of the
following:
(i) Upper urethral
and/or vaginal
mucosa, bladder
mucosa, rectal
mucosa, or fixed to
pelvic bone
(ii) Fixed or
ulcerated inguinofemoral lymph
nodes
IVB T
anyNanyM1 Any distant metastasis including
pelvic lymph nodes
TNM
Classification
T: Primary tumor N: Regional lymph nodes
(femoral and inguinal nodes)
T
x: Primary tumor cannot
be assessed
N
x: Regional lymph nodes cannot
be assessed
T0: No evidence of
primary tumor
N0: No regional lymph node
metastases
Tis: Carcinoma in situ
(preinvasive carcinoma)
N1: One or two regional lymph
node metastases with the
2707following features:
T1a: Lesions ≤2 cm in
size, confined to the vulva
or perineum and with
stromal invasion ≤1 mm
N1a: One or two lymph node
metastases each <5 mm
T1b: Lesions >2 cm in size
or any size with stromal
invasion >1 mm, confined
to the vulva or perineum
N1b: One lymph node metastasis
≥5 mm
T2: Tumor of any size
with extension to adjacent
perineal structures (lower
1/3 of urethra, lower of
1/3 vagina, anal
involvement)
N2: Regional lymph node
metastases with the following
features:
T3: Tumor of any size
with extension to any of
the following:
N2a: ≥3 lymph node metastases,
each <5 mm in diameter
upper 2/3 of urethra, upper
2/3 of vagina, bladder
mucosa, rectal mucosa, or
fixed to pelvic bone
N2b: ≥2 lymph node metastases
≥5 mm
M1: Distant metastasis
(including pelvic lymph
node metastasis)
N2c: Lymph node metastases with
extracapsular spread
N3: Fixed or ulcerated regional
lymph node metastasis
M: Distant metastasis
M0: No distant metastasis
aThe depth of stromal invasion is measured from the epithelial–stromal junction of the
adjacent most superficial dermal papilla to the deepest point of invasion.
FIGO, International Federation of Gynecology and Obstetrics; TNM, tumor node
metastasis.
FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the
vulva, cervix, and endometrium. Int J Gynecol Obstet 2009;105:103–104 (31); American
Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. Chicago, IL:
Springer New York, Inc.; 2010.
Table 40-5 Five-Year Survival for Patients With Vulvar Carcinoma (28)
FIGO Stage No. of Patients 5-Year Survival (%)
I 286 (34%) 79
II 266 (32%) 59
III 216 (26%) 43
IV 71 (8%) 13
FIGO, International Federation of Gynecology and Obstetrics.
Prognosis and Survival
Survival of patients with vulvar cancer correlates with FIGO stage (66). The
prognosis for patients with early-stage disease is generally good (Table 40-5).
The single most important prognostic factor is lymph node status (51–
53,56,67,68). A report from the Mayo Clinic on 330 patients with primary
squamous cell carcinoma of the vulva demonstrated a significant correlation
between lymph node status and risk of treatment failure, especially in the first 2
years following initial therapy: 44.2% overall recurrence rate with positive versus
17.5% with negative lymph nodes. [6] More than one-third of relapses
presented 5 years or more after initial therapy (69). There is a strong negative
correlation between the number of positive lymph nodes and survival (Table 40-6).
Patients with negative lymph nodes have a 5-year survival rate of over 80%;
for patients with positive nodes, 5-year survival falls below 50%. The number
of positive nodes is of critical importance: Patients with one microscopically
positive lymph node have a prognosis similar to those with all negative lymph
nodes, whereas patients with three or more positive nodes have a poor
prognosis and a 2-year survival rate of 20% (70). The survival rate for patients
with positive pelvic nodes is about 11% (71). In addition to the number of nodes
involved, the morphology of the positive groin nodes is of prognostic
significance. As demonstrated in several studies, significant negative predictors of
survival are the size of the nodal metastasis, the proportion of the node replaced
by tumor cells, and the presence of any extracapsular spread (59,67,72,73).
Histologic grade, tumor thickness, depth of stromal invasion, and lymph–vascular
space involvement contribute to the risk of lymph node involvement but are not
independent predictors of survival (70).
2709Post-treatment surveillance as recommended by the Society of Gynecologic
Oncology entails serial reviews of systems and careful physical examination with
annual cervical/vaginal cytology and imaging reserved for patients where findings
are concerning for recurrent disease (74). The frequency of surveillance visits
depends on the stage and recurrence risk:
Stage I, II disease every 6 months in years 1 and 2, and annually thereafter.
Stage III/IV disease every 3 months for the first two years, every 6 months
in years 3 to 5, and annually thereafter.
Table 40-6 Five-Year Survival for Patients With Vulvar Squamous Cell Carcinoma
by Number of Lymph Node Metastases (28)
No. of Lymph Node Metastases No. of Patients 5-Year Survival (%)
0 302 (61%) 81
1 66 (13%) 63
2 43 (9%) 30
3 24 (5%) 19
4 or more 62 (12%) 13
Treatment
[7] To individualize the patient’s care and determine the appropriate
therapy, it is necessary to independently manage the primary lesion and
groin lymph nodes using a separate incision technique for resection of the
vulvar lesion and for the node dissection. This is associated with lower
morbidity and metastases rarely occur in the skin bridge between vulva and
groin in patients without clinically suspicious inguinofemoral nodes (75,76).
Before initiation of therapy, all patients should undergo a careful clinical
examination, and colposcopy of the cervix, vagina, and vulva. Preinvasive (and
rarely invasive) lesions may be present at other sites along the lower genital tract,
and vulvar cancer is frequently multifocal. If disease is locally advanced, an
examination under anesthesia may be required to determine its full extent.
Imaging with PET/CT or MRI is performed for all but T1a lesions to aid in
assessment of the local disease and evaluate for metastases.
Management of the Primary Lesion
Microinvasive Vulvar Cancer (T1a)
2710[8] Tumors 2 cm or less in diameter with 1 mm or less invasion are
appropriately treated with a wide local excision, which is as effective as
radical surgery for the prevention of vulvar recurrences for these tumors
(77). The excision should go sufficiently deep into the dermis that depth of
invasion is fully assessed.
Early Vulvar Cancer (T1b)
[9] The modern approach to the management of patients with T1b
carcinoma of the vulva should be individualized. There is no standard approach
applicable to every patient, and emphasis is on performing the most conservative
operation that is consistent with cure of the disease through radical local excision
and surgical lymph node assessment while minimizing anatomic alterations and
disfigurement. An analysis of the literature indicates that the incidence of local
invasive recurrence after radical local excision or radical hemivulvectomy is not
higher than that after the historically performed radical vulvectomy (33,46,71,78–
80). In the presence of an otherwise normal-appearing vulva, radical local
excision is a safe surgical option, regardless of the size of the tumor or the
depth of invasion. Based on cumulative data of 413 patients reported in four
studies, an 8 mm or greater histopathologic resection margin results in a high rate
of local disease control (78,81–83). Of the 252 patients whose tumors were
resected with margins of 8 mm or greater, 2.4% experienced a local recurrence,
compared with 30.3% of the 161 patients whose margins were less than 8 mm.
Neither clinical tumor size nor the presence of coexisting benign vulvar pathology
correlated with local recurrence. It is important to bear in mind that paraffinembedded tissue shrinks by 20% to 25%. At the time of radical local excision,
at least a 1-cm grossly negative margin, without putting the skin under
tension, should be obtained and extended down to the level of the inferior
fascia of the urogenital diaphragm.
When vulvar cancer arises in the presence of VIN, lichen sclerosus, or some
nonneoplastic epithelial disorder, treatment is influenced by the patient’s age,
treatment history, and symptomatology. Elderly patients who often had many
years of chronic itching may not be disturbed by the prospect of a vulvectomy. In
younger women, it is desirable to conserve as much of the vulva as possible.
Radical local excision should be performed for the invasive disease, and the
associated intraepithelial disease should be treated in the manner most appropriate
to the patient. For example, topical steroids may be required for lichen sclerosus,
whereas VIN may require superficial local excision with primary closure,
ablation, topical therapy, or a combination thereof.
FIGURE 40-4 Small (T1) vulvar carcinoma at the posterior fourchette. (From Hacker NF,
Eifel PJ. Vulvar cancer. In: Berek JS, Hacker NF. Berek & Hacker’s Gynecologic
Oncology. 6th ed. Philadelphia, PA: Wolters Kluwer; 2015:574.)
Radical local excision is most appropriate for lesions on the lateral or
posterior aspects of the vulva (Fig. 40-4). Midline lesions pose special
challenges because of their proximity to clitoris, urethra, or anus. For anterior
lesions, conservative clitoris-sparing surgery allows for excellent local control as
2712long as pathologic margins are at least 8 mm (84). For tumors that involve the
clitoris or that are in close proximity to it, any type of surgical excision will have
psychosexual consequences. In addition, marked edema of the posterior vulva
may occur. For young patients with periclitoral lesions, the primary lesion can be
treated with a small field of radiation therapy with concomitant sensitizing
chemotherapy. Small vulvar lesions should respond very well to about 5,000 cGy
external radiation, and biopsy can be performed after therapy to confirm the
absence of any residual disease (58).
Early T2 Vulvar Cancer
[10] The indications for vulvar conservation can be extended to selected
patients with early T2 tumors. The tumor-free margin should be the same,
whether or not a radical vulvectomy or a radical local excision is performed.
It seems feasible and desirable to extend the indications for vulvar
conservation, particularly for younger patients. Tumors that are suitable for
a conservative resection are those involving the posterior vulva and lower
vagina, where preservation of the anus, clitoris, and urethra is feasible.
For patients with more advanced T2 lesions, management consists of radical
vulvectomy and/or chemoradiation therapy. When the disease involves the distal
urethra or anus, partial resection of these organs would be required. Thus, it is
often preferable to give preoperative radiation therapy with chemosensitization to
allow for a less radical resection depending on location and response.
Closure of Large Defects
After radical local excision, primary closure without tension can be
accomplished for smaller defects. If an extensive dissection is required to
treat a large primary lesion, a number of options are available to repair the
defect based on the defect location, dimension, patient age, and
comorbidities:
1. An area may be left open to granulate, which it will usually do over a period of
6 to 8 weeks (85); this may be hastened by the application of vacuum-assisted
wound closure and negative pressure dressings (86).
2. Full-thickness rotational or advancement fasciocutaneous flaps may be
devised, such as the rhomboid flap, the mons pubis pedicle flap, the pudendal
thigh flap, or the V-Y-advancement flap (87–91). Fasciocutaneous flaps are
reliable and can be transferred as pedicle or island flaps (92). For the lower
and mid vulva, a V-Y gluteal fold flap may be preferred because of the good
esthetic outcome and well-concealed donor scar (91,93).
3. A broad range of myocutaneous flaps have been used for vulvar reconstruction
2713to cover large defects. These include unilateral or bilateral gracilis
myocutaneous grafts or a vertically oriented rectus abdominis muscle flap.
Because the graft brings a new blood supply to the area, it is particularly
applicable if the vulva is poorly vascularized from prior surgical resection or
radiation (91,93,94). Disadvantages include complexity and duration of the
procedure, the bulkiness of the flap, and donor-site sequelae.
Advanced Disease: Large T2 and T3 Primary Tumors
Treatment of locally advanced vulvar cancer remains challenging. To achieve
primary surgical clearance for tumors involving the upper urethra, anus, rectum,
or rectovaginal septum, pelvic exenteration is needed in addition to radical
vulvectomy and inguinal–femoral lymphadenectomy, which carries an extremely
high physical and psychological morbidity (95,96). Reported 5-year survival rates
with this approach are about 50% (97–99), with survival being significantly
determined by lymph node status. A series including 57 patients undergoing
primary anovulvectomy reported a median survival of 69 months with 59% of
patients experiencing one or more postoperative complications and 33% requiring
adjuvant therapy (100). For many of these patients, a combined approach of
chemoradiation therapy with or without subsequent surgery offers equal or
improved survival with reduced morbidity and may be the preferred treatment
approach. Numerous small prospective and retrospective series report on the use
of external beam radiation with concomitant chemotherapy to shrink the primary
tumor. Reported initial response rates to chemoradiation are 80% to 90%, and
operability is achieved in 63% to 92% of cases (101–109). This chemoradiation is
followed by a limited resection of the tumor bed on an individualized basis. Case
series suggest that approximately one-half of the specimens will contain residual
tumor, and local relapse rates are as high as 50% with external chemoradiation
alone (110). The GOG phase II study using a combination of weekly cisplatin
with radiation followed by surgical resection of gross residual disease, or biopsy
if clinically no residual cancer, resulted in a complete clinical response in 64% of
patients, with biopsy showing complete pathologic response in 50% of patients
without the need for posttreatment surgery in this subset of patients (111).
As experience with this combination therapy evolved, it appeared that external
beam therapy is appropriate for most cases, with more selective use of
brachytherapy. The extensiveness of the surgery is significantly modified. A
limited vulvar resection is advocated, and bulky N2 and N3 nodes are
resected without full groin lymphadenectomy to avoid the leg edema
associated with groin lymphadenectomy and radiation. With this combined
radiation–surgical approach, 5-year survival rates as high as 76% are
reported. With the experience accrued, preoperative radiation with concurrent
2714chemotherapy is regarded as the first treatment of choice for patients with
advanced vulvar cancer who would otherwise require some type of pelvic
exenteration or stoma. Neoadjuvant therapy is not justified in patients with tumors
that can be adequately treated with primary radical vulvectomy and bilateral groin
node dissection.
Management of the Lymph Nodes
Appropriate assessment for nodal involvement and groin dissection is the
single most important factor in decreasing the mortality from early vulvar
cancer. A careful examination of the groin is carried out as the presence of
clinically palpable inguinofemoral lymph nodes is an important determinant of
management.
When assessing a patient for groin dissection, the following facts should to be
kept in mind:
1. The only patients with virtually no risk of lymph node metastases are those
whose tumor is small (≤2 cm) and invades the stroma to 1 mm or less (T1a).
2. Patients who develop recurrent disease in an undissected groin have a greater
than 90% mortality (111).
3. Based on the laterality of the vulvar lesions and the status of the ipsilateral
groin, an ipsilateral or bilateral lymphadenectomy becomes necessary.
FIGURE 40-5 Skin incision for groin dissection through a separate incision. A line is
drawn 1 cm above and parallel to the groin crease, and a narrow ellipse of skin is removed.
(Revised with permission from Berek JS, Hacker NF. Practical Gynecologic Oncology.
2nd ed. Baltimore, MD: Williams & Wilkins; 1994:418.)
Groin dissection is associated with postoperative wound infection and
breakdown. Although the incidence of wound breakdown is reduced significantly
when separate incisions are used for the groin dissection and drains are left in situ
until daily output is below 25 to 30 cc, lymphocyst formation and chronic leg
edema remain a major problem (Fig. 40-5) (77).
All patients whose tumors demonstrate more than 1 mm of stromal
invasion or whose tumors are larger than 2 cm (T1b and above) require
inguinal–femoral lymphadenectomy. If there is any question regarding the need
for inguinofemoral lymphadenectomy, a Keys biopsy or wedge biopsy of the
primary tumor should be obtained, and the depth of invasion should be
determined. If it is smaller than 1 mm on the wedge biopsy specimen, the entire
lesion should be locally excised and analyzed histologically to determine the
depth of invasion. If the lesion is 2 cm in diameter or smaller and there is no
invasive focus larger than 1 mm, groin dissection may be omitted, provided there
2716is no lymph–vascular space invasion and there are no clinically suspicious groin
lymph nodes. An occasional patient with less than 1 mm of stromal invasion has
documented groin node metastases, but the incidence is so low that it is of no
practical significance (112,113).
Inguinal–Femoral Lymphadenectomy
If groin dissection is indicated in patients with vulvar cancer, it should be a
thorough inguinal–femoral lymphadenectomy. The GOG reported six groin
recurrences among 121 patients with tumors 2 cm or less after a superficial
(inguinal) dissection, although the removed inguinal nodes were negative, and a
study from the M. D. Anderson Cancer Center reported a 9% groin recurrence
rate in 104 patients with vulvar cancer and negative nodes on superficial inguinal
lymphadenectomy (38,39). Although it is unclear whether all of these recurrences
were in the femoral nodes, both studies indicate that an incomplete groin
dissection will increase the number of groin recurrences and mortality. GOG data
indicate that radiation therapy cannot substitute for groin dissection followed by
selective radiation as indicated, even in patients with clinically nonsuspicious
lymph nodes (114). This GOG study was closed early because a significantly
higher incidence of recurrences occurred in women who were receiving groin
radiation therapy only (19% vs. 0%). The dose of radiation was 5,000 cGy given
in daily 200-cGy fractions to a depth of 3 cm below the anterior skin surface.
Although the radiation regimen prescribed was criticized extensively, other
uncontrolled studies give no evidence for better groin control with radiotherapy
(115,116). Surgery remains the treatment of choice for the groin for women with
vulvar cancer. Vulvar cancer patients with T2 or T3 lesions scheduled for primary
or neoadjuvant chemoradiation should undergo an inguinofemoral
lymphadenectomy prior to initiation of chemoradiation, given the lower survival
with primary groin radiation compared with groin dissection followed by
radiation (115). Some patients with T1b and T2 tumors and no palpable groin
lymph nodes on examination are candidates for sentinel lymph node assessment.
Unilateral Versus Bilateral Groin Dissection
It is not necessary to perform a bilateral groin dissection if the primary
lesion is unilateral and the ipsilateral lymph nodes are negative. In a patient
with a unilateral lesion and negative ipsilateral groin nodes, the risk of
contralateral lymph node metastasis is very low (33,45). In a study from the Mayo
Clinic, 8 of 163 patients with unilateral vulvar cancers (4.8%) had bilateral lymph
node metastases and only 3 (1.8%) had isolated contralateral lymph node
metastases. None of the patients with unilateral vulvar lesions that were either 2
cm or less or had 5 mm or less depth of invasion had bilateral groin node
2717involvement at diagnosis (45). There is an increase in the risk of contralateral
nodal involvement proportional to the number of positive ipsilateral inguinal
nodes (45,57). It is recommended that patients with any bulky or multiple
microscopically positive ipsilateral groin lymph nodes undergo contralateral
inguinal–femoral lymphadenectomy. Bilateral inguinal–femoral
lymphadenectomy should be performed for midline lesions (clitoris, anterior
labia minora, posterior fourchette) or those within 2 cm of the midline
because of the more frequent contralateral lymph flow from these regions
(117).
Management of Bulky Groin Nodes
All clinically or radiologically suspicious groin nodes should be resected if
deemed resectable and the patient is a surgical candidate. If nodal metastasis is
confirmed by preoperative biopsy or intraoperative frozen section, a full
inguinofemoral lymphadenectomy may be omitted to decrease morbidity with
subsequent radiation without compromising survival (118,119). If groin lymph
nodes are palpably enlarged, but frozen section does not demonstrate metastatic
disease, a complete inguinofemoral lymphadenectomy should be performed.
Patients with fixed, unresectable groin nodes should be treated with primary
chemoradiation. If there is no other evidence of metastatic disease following
chemoradiation, GOG data suggest that it may be appropriate to resect the
residual nodes (120).
Management of Pelvic Lymph Nodes
In the past, pelvic lymphadenectomy was part of the routine surgery for invasive
vulvar cancer. The incidence of pelvic lymph node metastasis is rare in the
absence of groin node involvement, and a more selective approach is preferred
(Table 40-2). Patients most prone to pelvic lymph node metastasis are those with
three or more pathologically positive groin nodes (30,42,54,121). In addition to
the number of nodes involved, the morphology of the positive groin nodes is of
prognostic significance. As demonstrated in several studies, significant negative
predictors of survival are the number of positive nodes, the size of the nodal
metastasis, the proportion of the node replaced by tumor cells, and the presence of
any extracapsular spread (63,67,72,73). In these patients, the pelvis requires
treatment by radiation. If a preoperative pelvic imaging study reveals bulky
pelvic lymph nodes, resection of these nodes should be performed via an
extraperitoneal approach prior to radiation because of the limited ability of
external beam radiation therapy to sterilize bulky positive pelvic nodes.
Sentinel Lymph Node Studies
Increasing evidence supports the use of intraoperative lymphatic mapping using
2718lymphoscintigraphy with technetium-99m-labeled nanocolloid and isosulfan blue
dye to identify a sentinel node that would predict the presence or absence of
regional nodal metastases. The strong interest in the sentinel node concept lies in
the desire to reduce the significant lifelong morbidity of lymphedema associated
with a thorough inguinofemoral lymphadenectomy, as only 25% to 35% of
patients with early-stage vulvar cancer will have lymph node metastases and thus
benefit from the procedure. Reliable identification of the sentinel node and
forgoing full lymphadenectomy in patients with clinically nonsuspicious groin
lymph nodes and a negative sentinel node significantly reduces the number of
patients who undergo unnecessary, extensive lymphadenectomy in the absence of
disease. This is contingent upon a negative sentinel lymph node reliably
predicting the absence of any other nodal metastases given the greater than 90%
mortality associated with a groin recurrence.
The sentinel lymph node procedure has led to a major decrease in
morbidity compared with a complete inguinofemoral lymphadenectomy
without negatively impacting prognosis in select patients (122–126). In
experienced hands, this procedure is reliable and suitable in early-stage
disease with unifocal tumor lesions less than 4 cm in size and clinically
negative lymph nodes on groin examination and imaging. A Cochrane
Database review of 34 studies evaluating 2,396 groins in 1,614 women reported
for blue dye plus technetium combined a sentinel node detection rate of 98% with
a pooled sensitivity estimate of 0.95 (95% CI 0.89 to 0.97), and a negative
predictive value of greater than 95% (127). The combination of blue dye and
radiocolloid is superior over blue dye alone for sentinel node identification.
Ultrastaging techniques such as serial micro-sectioning and
immunohistochemistry staining are used to detect micrometastases in the sentinel
node if initial hematoxylin and eosin section is negative. This increases the
detection rate of lymph node metastasis (123).
Recurrence in the groin is fatal in over 90% of patients. Thus, groin
recurrence is ultimately a critical outcome measure for surgical groin assessment
techniques. A meta-analysis of recurrence rate included 23 studies and
demonstrated a significantly higher groin recurrence rate with superficial inguinal
lymphadenectomy (6.6%; 95% CI 4.4–9.0) than with complete inguinofemoral
lymphadenectomy (1.4%; 95% CI 0.4–2.9) with the recurrence rate for patients
undergoing the sentinel node procedure being 3.4% (95% CI 1.8–5.4) (128).
Long-term follow-up of the GROINSS-V study, one of the largest studies on the
safety of the sentinel node procedure in vulvar cancer, reported for 377 patients
with unifocal <4 cm T1 vulvar cancer a local recurrence rate of 27.2% at 5 years
and 39.5% at 10 years. Isolated groin recurrences occurred in 2.5% of patients
with negative sentinel nodes and 8% of patients with positive sentinel nodes. All
2719groin recurrences occurred within 25 months of primary treatment (129). Some
small series of sentinel nodes in vulvar cancer with inclusion criteria similar to
those in GROINSS-V showed unexpectedly high false-negative rates of 8% to
27%, some of which may be attributable to patient selection and insufficient
experience of low-volume providers. This highlights the prerequisites for highquality sentinel node procedures, which include careful patient selection, trained
personnel, and appropriate infrastructure. The technique might otherwise carry
with it an unjustifiable rise in the frequency of groin recurrences.
Postoperative Management
Despite the age and general medical condition of many elderly patients with
vulvar cancer, surgery is usually remarkably well tolerated. Patients should be
able to commence eating a low-residue diet on the first postoperative day. In the
past, bed rest was advised for 3 to 5 days postoperatively to allow for
immobilization of the wounds and to foster healing. Because radical local
excisions are being performed with increasing frequency and groin
lymphadenectomy is done through separate incisions, patients begin
ambulation on postoperative day 1 or 2. Pneumatic calf compression and
subcutaneous low molecular weight heparin should be given to help prevent deep
venous thrombosis, and active leg movements are to be encouraged. Frequent
dressing changes are performed to keep the vulvar wound dry. Meticulous
perineal hygiene is maintained. Suction drainage of each side of the groin is
continued until output is minimal to help decrease the incidence of groin seromas.
It is not uncommon for suction drainage to continue for 10 or more days. The
Foley catheter is removed when the patient is ambulatory. If there is significant
periurethral swelling, prolonged bladder drainage may be advisable. If there is
breakdown of the vulvar wound, sitz bath or whirlpool therapy is helpful,
followed by drying of the perineum with a hair dryer.
Early Postoperative Complications
The major immediate morbidity is related to groin wound infection, necrosis, and
breakdown. This complication used to be reported in as many as 53% to 85% of
patients having an en bloc operation (29,30). With the separate-incision
approach, the incidence of wound breakdown has been reduced to approximately
44%; major breakdown occurs in about 14% of patients (31,75,130,131).
With appropriate antibiotics, debridement, and wound dressings, the area will
granulate and re-epithelialize over several weeks and may be managed with home
nursing. Whirlpool therapy is effective for areas of extensive breakdown. The
most common complication with the separate incision approach continues to be
wound infection requiring antibiotic therapy and lymphocyst formation, reported
2720in up to 40% of cases (131). The GROINSS-V study reported wound break down
of the groin in 34% of patients undergoing complete inguinofemoral
lymphadenectomy and 12% of patients undergoing the sentinel lymph node
procedure with respective cellulitis rates of 21% and 5% (122). Symptomatic
lymphocysts should be managed by periodic sterile aspiration.
Other early postoperative complications include urinary tract infection, seromas
in the femoral triangle, deep venous thrombosis, pulmonary embolism,
myocardial infarction, hemorrhage, and, rarely, osteitis pubis. Anesthesia of the
anterior thigh resulting from femoral nerve injury is common and usually resolves
slowly.
Late Complications
One major late complication is chronic lymphedema, which occurs in about
30% of patients (29–31,130–132). Recurrent lymphangitis or cellulitis of the leg
develops in about 10% of patients and usually responds to oral antibiotics. The
GROINSS-V study, prospectively following 403 patients and 623 groin
evaluations, reports lymphedema in 25% of patients undergoing complete
inguinofemoral lymphedema and 2% of patients undergoing the sentinel lymph
node procedure with respective recurrent erysipela rates of 16% and 0.4% (122).
Urinary stress incontinence, with or without genital prolapse, occurs in about 10%
of patients after radical vulvectomy and may require corrective surgery. Introital
stenosis can lead to dyspareunia and may require a vertical relaxing incision,
which is sutured transversely. An uncommon late complication is femoral hernia,
which can be prevented intraoperatively by closure of the femoral canal with a
suture from the inguinal ligament to Cooper’s ligament. Pubic osteomyelitis and
rectovaginal or rectoperineal fistulas are rare late complications.
Other major long-term treatment complications associated with the extent
of vulvar surgery include depression, altered body image, and sexual
dysfunction (95,96). Modifications in the radical extent of the surgical approach
and appropriate preoperative and postoperative counseling may help lessen some
of the psychological trauma.
Role of Radiation Therapy
Radiation therapy traditionally had a limited role in the management of patients
with vulvar cancer. In the orthovoltage era, local tissue tolerance was poor and
vulvar necrosis was common, but with megavoltage therapy, tolerance improved
significantly. Radiation therapy, frequently with concurrent chemotherapy, has an
increasingly important role in the management of patients with vulvar cancer. It is
important to remember that, with a rare exception, radiation therapy alone has
little place in the primary management of vulvar cancer. It is primarily indicated
2721in conjunction with surgery.
The indications for radiation therapy for patients with primary vulvar cancer
are evolving. Radiation is indicated in the following situations:
1. Preoperative chemoradiation in patients with advanced disease who would
otherwise require pelvic exenteration or suffer loss of anal or urethral
sphincteric function.
2. Preoperative chemoradiation in patients with fixed, unresectable groin nodes.
3. Postoperatively, to treat the pelvic lymph nodes and groins of patients with
multiple microscopically positive groin nodes, one or more macrometastasis
(10 mm or larger), or any evidence of extracapsular spread.
Possible indications for radiation therapy to the vulva include the following:
1. Postoperatively, to help prevent local recurrences in patients with involved (or
close) surgical margins. Radiation to the primary tumor bed following
resection with involved margins may improve 5-year overall survival to 67%,
which is comparable to that of patients with negative margins (133,134).
2. As primary therapy for patients with small primary tumors, particularly clitoral
or periclitoral lesions in young and middle-aged women, for whom surgical
resection would have significant psychological consequences (135).
The benefit to adjuvant radiation therapy in patients with a single groin
micrometastasis is unclear. While the prognosis appears somewhat worse for
this group of patients compared with node negative patients (126,136), there is
little evidence of benefit to adjuvant radiation therapy (54,136,137). Thus, no
additional treatment and careful observation is an option if one microscopically
positive groin node (5 mm or less tumor deposit) is found in a fully dissected
groin. For patients with a single groin node metastasis, a retrospective SEER
database review suggests that adjuvant radiation may provide a therapeutic
benefit, especially if the groin dissection was limited (138). However, size and
characteristics of the nodal metastasis were not known. If unilateral groin
dissection was performed for a lateral lesion, there seems to be no indication for
dissection of the other side, because contralateral lymph node involvement is
likely only if there are multiple microscopic or any gross ipsilateral inguinal node
metastases (54,57). If clinically evident groin metastases, any extracapsular
spread, or two or more microscopically positive groin nodes are found, the
patient is at increased risk of groin and pelvic recurrence and should receive
postoperative groin and pelvic irradiation. In 1977, the GOG initiated a
prospective trial in which patients with positive groin nodes were randomized to
either ipsilateral pelvic node dissection or bilateral pelvic plus groin irradiation
2722(57). The survival rate for the radiation group (68% at 2 years) was significantly
better than the survival rate for the pelvic lymphadenectomy group (54% at 2
years) (p = 0.03). The survival advantage was limited to patients with clinically
evident groin nodes or more than one microscopically positive groin node. Groin
recurrence occurred in 3 of 59 patients (5%) treated with radiation, compared with
13 of 55 (23.6%) patients treated with lymphadenectomy (p = 0.02). Four patients
who received radiation had a pelvic recurrence, compared with one who had
lymphadenectomy. These data highlight the value of groin irradiation in
preventing groin recurrence in patients with multiple positive groin nodes. This
concept of adjuvant radiation therapy including bilateral groins and pelvis for
patients with two or more positive nodes is further supported by the contemporary
AGO-CaRE-1 study demonstrating better outcomes with this adjuvant treatment
approach (136).
Recurrent Vulvar Cancer
Recurrence of vulvar cancer correlates closely with the number of positive groin
nodes (54). Patients with fewer than three positive nodes, particularly if the nodes
are only microscopically involved, have a lower incidence of recurrence at any
site, whereas patients with three or more positive nodes have a high incidence of
local, regional, and systemic recurrences (54,57).
Most recurrences of vulvar cancer occur within the first 2 years from
initial therapy, with groin recurrences occurring sooner (median time to
recurrence 6 to 7 months) than vulvar recurrences (median time to
recurrence 3 years) (69,139–141). About one-third of vulvar cancer relapses
present 5 or more years after initial therapy (69). In a long-term follow-up
study at the Mayo Clinic, nearly 1 in 10 patients with vulvar cancer had a late
(longer than 5 years) reoccurrence of disease (69). More than 95% of those late
relapses had local reoccurrences (same site recurrence or second primary vulvar
site). Because of this propensity for late local reoccurrence, regular and long-term
careful examinations of the vulva and groin constitute the cornerstone of
posttreatment surveillance for these patients.
The published literature on the management and outcome of recurrent disease
is limited. The timing and primary site of recurrence is critical to the prognosis
postrecurrence. Although groin recurrences tend to occur early and are nearly
always fatal, 5-year overall survival rates of 50% to 70% are reported for patients
with surgically treated isolated vulvar recurrences and more than 60% of patients
with local recurrence or reoccurrence were alive at 20 years in the Mayo Clinic
long-term follow-up study (69,141,142). In contrast, a series of 502 patients
demonstrate the poor prognosis for patients with groin, pelvic, or distant
recurrences: 5-year survival rates of 27% with inguinal or pelvic recurrence, 15%
2723with distant recurrence, and 14% with multiple sites of recurrence (143).
Management of recurrent vulvar cancer depends on site of recurrence, prior
treatments, and patient performance status.
Local Vulvar Recurrence
Margin status at the time of radical resection of the vulvar cancer is the most
powerful predictor of local vulvar recurrence, with an almost 50%
recurrence risk with margins closer than 0.8 cm (81). Margin status does not
predict survival (68). Local vulvar recurrences are more likely in patients with
primary lesions larger than 4 cm in diameter, especially if lymph–vascular space
invasion is present, and in patients with deeply invasive tumors (133,144,145).
When detected early, isolated local failure is usually treatable by additional
surgical therapy, often with a myocutaneous graft to cover the defect
(31,38,46,75,146). Radiation therapy, particularly a combination of external beam
therapy plus interstitial needles, frequently combined with chemotherapy, is used
to treat vulvar recurrences (147).
Three distinct patterns of local recurrence are described: remote vulvar
recurrence (greater than 2 cm from the primary tumor site), primary tumor site
recurrence (within 2 cm of the primary tumor site), and skin bridge recurrence
(78,145). Although reported treatment outcomes are excellent for remote site
recurrences with 3-year survival rates of 67% to 100%, the literature is
controversial as to the prognostic significance of primary tumor site recurrences,
with one study reporting a 3-year survival rate of only 15% and the other a 5-year
survival of 93% (78,145). Patients with skin bridge recurrence have a very poor
prognosis, similar to those with groin recurrence.
Regional Inguinal and Distant Recurrence
Regional and distant recurrences are difficult to manage and are associated
with a poor prognosis (133,139,140). Radiation therapy may be used in
conjunction with surgery for groin recurrence, and chemotherapeutic agents that
have activity against squamous carcinomas may be offered for distant metastases.
The literature on the use of chemotherapy for recurrent vulvar cancer consists
mainly of small series. The most extensively studied regimens contain bleomycin,
methotrexate, and lomustine (a nitrosourea); bleomycin and mitomycin C; or
cisplatin, vinorelbine, and paclitaxel, but response rates are low and the duration
of response is usually disappointing (148–152). Extrapolating from data for the
management of recurrent cervical cancer, many experts recommend the
administration of platinum/taxane-based chemotherapy with or without
bevacizumab. There is a role for palliative radiation of select symptomatic
recurrences. Long-term survival is very uncommon with regional or distant
2724recurrence. Symptom control and quality of life are important treatment goals,
and early involvement of a multidisciplinary palliative care team is generally
indicated.
MELANOMA
Vulvar melanomas are rare, with an incidence of 0.1 to 0.19 per 100,000
women (153,154). They account for approximately 10% of all cases and are
the second most common form of vulvar malignancy. Most melanomas arise
de novo, but they may arise from a preexisting junctional nevus. Vulvar
melanomas occur most frequently in postmenopausal white women, but are
commonly seen in individuals with darker pigmented skin. Contrary to cutaneous
melanoma, based on SEER data, there are low racial differences in vulvar
melanomas, the development of which appears to be determined by factors other
than ultraviolet radiation exposure and the photoprotective effects of melanin seen
in cutaneous melanoma (155). The incidence of cutaneous melanomas worldwide
is increasing significantly, but not that of vulvar melanoma (155). Vulvar
melanomas appear to behave in a manner similar to that of other truncal
cutaneous melanomas. From a molecular genetic perspective, vulvar melanoma
more closely resembles acral lentiginous melanoma rather than cutaneous
melanoma. KIT is the most commonly mutated gene detected in up to 35% of
vulvar melanomas. More rarely NRAS and BRAF mutations have been described
(156).
FIGURE 40-6 Melanoma of the vulva involving the right labium minus. (From Hacker
NF, Eifel PJ. Vulvar cancer. In: Berek JS, Hacker NF. Berek & Hacker’s Gynecologic
Oncology. 6th ed. Philadelphia, PA: Wolters Kluwer; 2015:595.)
Most patients with vulvar melanoma have no symptoms other than a
pigmented lesion that may be enlarging. The lesion may present as macules,
papules, or nodules of irregular configuration and coloration, typically greater
than 7 mm in diameter. Some patients have pruritus or bleeding, a few have a
groin mass, and some lesions are amelanotic. Vulvar melanomas occur most
frequently on the labia majora, followed by the labia minora and the clitoris (157)
(Fig. 40-6); extension into the urethra or vagina at discovery is not uncommon.
Any pigmented lesion on the vulva should be excised or, if the lesion is large,
sampled for biopsy unless it is known to have been present and unchanged for
some years. Most vulvar nevi are junctional and may be precursor lesions to
melanoma; any nevus of the vulva should be removed. Excisional biopsy, rather
than a shave, wedge, or punch biopsy, is recommended to ensure evaluation
of the entire lesion and facilitate microstaging and measurement of tumor
thickness.
Histopathology
2726There are three basic histologic types of vulvar melanoma (158; Fig. 40-7):
1. The mucosal lentiginous melanoma (25% to 57%) is a flat freckle that may
become quite extensive but tends to remain superficial.
2. The nodular melanoma (22% to 28%), which is the most aggressive, is
characterized by a raised lesion that penetrates deeply and may metastasize
widely.
3. The superficial spreading melanoma (4% to 56%) tends to remain relatively
superficial early in its development.
In one of the larger reported series, more than one-fourth of the cases of
melanomas were macroscopically amelanotic (153). Vulvar melanoma tends to
spread early, lymphatically and hematogenously.
Staging
The FIGO staging used for squamous lesions is not applicable to melanomas
because the lesions are usually much smaller and the prognosis is related to
tumor thickness rather than to the diameter of the lesion. The vulvar skin
lacks a well-defined papillary dermis. Breslow measured the thickest portion of
the melanoma from the surface of intact epithelium to the deepest point of
invasion (159). It is important to recognize that tumor thickness and lymph node
status are the primary determinants of survival. The AJCC staging guidelines
for cutaneous melanoma (160) should be used for vulvar melanoma.
Validation studies of staging systems for vulvar melanoma have confirmed that
survival is best predicted by the AJCC staging system (161,162). The AJCC
staging system for cutaneous melanoma is shown in Table 40-7.
The revised 2017 eighth edition of the AJCC staging for cutaneous melanoma
reflects that tumor thickness remains the primary determinant of the T staging in
addition to the presence of ulcerations (160). The mitotic rate is no longer a
staging criterion for T1 tumors, but it remains an important prognostic factor and
should be recorded for all patients with a T1 to T4 primary melanoma lesion.
Specific immunohistochemistry criteria for the detection of micrometastases are
included. Regional lymph node involvement is classified as either clinically
occult (found microscopically, usually based upon a sentinel lymph node biopsy)
or clinically detected (on physical examination or by imaging) without associated
specific size criteria. Microsatellites, satellites, and in-transit cutaneous and/or
subcutaneous metastases are included to stratify the lymph node staging. The
site(s) of distant metastatic disease as stratified by serum lactate dehydrogenase
levels remain part of the staging.
FIGURE 40-7 Vulvar melanoma. Spindle-shaped melanoma cells form interlacing
bundles, and some contain melanin pigment (right upper corner). Epidermal invasion is
evident in the form of Pagetoid migration (left upper corner).
Treatment
Vulvar melanomas are rare and there is minimal data specific to the treatment of
vulvar melanoma. With better understanding of the prognostic significance of the
microstage, some individualization of treatment developed. When feasible, the
primary treatment modality for vulvar melanoma is excision. It is accepted that
lesions with less than 1 mm of invasion may be treated with radical local
excision alone. With more invasive lesions, radical vulvectomy with bilateral
inguinofemoral lymphadenectomy has historically been the recommended
treatment (163). Extrapolating from cutaneous melanoma and supported by
smaller case series of the vulva, it has become apparent that most failures are
distant and radical vulvectomy does not appear to enhance survival. Based on this
recognition, the contemporary more conservative surgical approach evolved,
where surgery for vulvar melanoma entails a radical local excision with
sentinel lymph node biopsy in clinically node negative patients, followed by
2728complete inguinofemoral lymphadenectomy for those with involved sentinel
lymph nodes. Skin margins for melanomas <1 mm thick should be 1 cm; if
anatomically feasible, margins should be extended to 2 cm for melanomas with
≥1 mm thickness. At least a 1-cm tumor-free deep surgical margin down to the
fascia is recommended, irrespective of tumor thickness. This conservative
surgical approach is further supported by a SEER data analysis of 644 patients
with vulvar melanoma which did not find a survival difference in patients with
localized disease treated with more conservative versus radical surgery. Five-year
disease-specific survival rates for patients undergoing conservative surgery for
localized disease were 75% versus 79% for those undergoing radical surgery
(164). Because melanomas commonly involve the clitoris and labia minora, the
vaginourethral margin of resection is a common site of failure, and care should be
taken to obtain an adequate “inner” resection margin (165). A 10-year survival
rate of 61% was shown for lateral lesions, compared with 37% for medial lesions
(p = 0.027) (144). In locally advanced cases, primary exenteration should not be
routinely offered because of the high risk of distant failure. In these cases,
radiation therapy with or without immunotherapy may be a preferred
consideration.
Controversy exists as to which patients may benefit from inguinal–femoral
lymphadenectomy. A prospective study by the GOG demonstrated that the risk
of inguinal–femoral lymph node metastasis correlated with the Breslow
microstage (166). As with cutaneous melanoma, it appears that for superficial
lesions (tumor thickness <1 mm), the risk for nodal spread is so low that routine
lymphadenectomy is not indicated as long as the nodes appear clinically free of
disease. For intermediate-thickness (1 to 4 mm) cutaneous melanoma, a
randomized controlled trial of elective lymph node dissection versus observation
showed a 5-year survival advantage for patients who underwent elective lymph
node dissection, who were younger than 60 years, and whose tumors were
characterized by 1- to 2-mm thickness and no ulcerations (167). Patients with
deeply invasive cutaneous melanomas (≥4 mm tumor thickness) have a high risk
of regional and systemic metastases and were noted to be unlikely to benefit from
regional lymphadenectomy (168). A randomized controlled trial evaluated 2,001
patients with intermediate thickness cutaneous melanoma and clinically negative
nodes who underwent either nodal observation with lymphadenectomy for local
relapse or sentinel node biopsy with immediate complete lymphadenectomy for
those with positive sentinel nodes. Significantly improved melanoma-specific 10-
year survival rates were observed in the sentinel node assessment group (169).
Specific to patients with vulvar melanoma, there is a small body of literature to
suggest that there may be a clinical benefit in elective groin lymphadenectomy
and the resection of clinically positive nodes (170,171). Studies on the sentinel
2729lymph node procedure specific to vulvar melanoma are limited. Although the
sentinel node procedure appears appropriate for select patients with vulvar
melanoma, the importance of an expert team familiar with the procedure and
consisting of a gynecologic oncologist, a nuclear medicine specialist, and a
pathologist with expertise in microstaging cannot be overemphasized. The
prognosis for patients with positive pelvic nodes is so poor that there seems to be
no value in performing pelvic lymphadenectomy for this disease.
Table 40-7 Eighth (2017) Edition AJCC Melanoma TNM Definitions and Staging
(161)
Primary Tumor
Tx Primary tumor thickness cannot be assessed
T0 No evidence of primary tumor
Tis Melanoma in situ
T1 ≤1 mm thickness
T1a a: <0.8 mm and without ulceration
T1b b: 0.8–1 mm or with ulceration
T2 >1 to 2 mm thickness
T2a a: without ulceration
T2b b: with ulceration
T3 >2 to 4 mm thickness
T3a a: without ulceration
T3b b: with ulceration
T4 >4 mm thickness
T4a a: without ulceration
T4b b: with ulceration
Regional Lymph Nodes
NX Regional nodes not assessed
2730N0 No regional metastases detected
N1 One tumor-involved node or in-transit, satellite, and/or microsatellite
metastases with no tumor-involved nodes
N1a a: one clinically occult (i.e., detected by SLN biopsy)
N1b b: one clinically detected (on examination or imaging)
N1c c: in-transit, satellite, and/or microsatellite metastases with no tumorinvolved nodes
N2 Two or three tumor-involved nodes or in-transit, satellite, and/or
microsatellite metastases with one tumor-involved node
N2a a: two or three clinically occult (i.e., detected by SLN biopsy)
N2b b: two or three positive, at least one clinically detected
N2c c: in-transit, satellite, and/or microsatellite metastases with one tumorinvolved node (occult or clinically detected)
N3 Four or more tumor-involved nodes or in-transit, satellite, and/or
microsatellite metastases with two or more tumor-involved nodes, or
any number of matted nodes without or with in-transit, satellite, and/or
microsatellite metastases
N3a a: four or more clinically occult (i.e., detected by SLN biopsy)
N3b b: four or more, at least one clinically detected, or any number of matted
nodes
N3c c: in-transit, satellite, and/or microsatellite metastases with two or more
tumor-involved nodes (occult or clinically detected) or any matted
nodes
Distant Metastases
M0 No evidence of distant metastasis
M1 Evidence of distant metastasis
M1a a: Distant metastasis to skin, soft tissue including muscle, and/or
nonregional lymph node
M1a(0) a(0): LDH not elevated
2731M1a(1) a(1): LDH elevated
M1b b: Distant metastasis to lung with or without M1a sites of disease
M1b(0) b(0): LDH not elevated
M1b(1) b(1): LDH elevated
M1c c: Distant metastasis to non-CNS visceral sites with or without M1a or
M1b sites of disease
M1c(0) c(0): LDH not elevated
M1c(1) c(1): LDH elevated
M1d d: Distant metastasis to CNS with or without M1a, M1b, or M1c sites of
disease
M1d(0) d(0): LDH not elevated
M1d(1) d(1): LDH elevated
Stage
0 Tis, N0, M0
IA T1a, N0, M0
IB T1b or T2a, N0, M0
IIA T2b or T3a, N0, M0
IIB T3b or T4a, N0, M0
IIC T4b, N0, M0
III Any T, ≥N1, Mo
IV Any T, Any N, M1
SLN, sentinel lymph node.
Management of Patients With Nodal Metastases
The decision whether to recommend adjuvant therapy depends upon the stage at
diagnosis, in the context of patient age, comorbidity, and patient preferences. The
presence of lymph node involvement is associated with a significant increase in
risk of recurrence. Adjuvant immunotherapy with nivolumab, a programmed cell
2732death protein 1 (PD-1) inhibitor, is frequently recommended for patients with
lymph node involvement. Nivolumab is more effective and less toxic than
ipilimumab, which targets cytotoxic T-lymphocyte– associated protein 4 (CTLA-
4) (172). In BRAF wild-type patients, ipilimumab is associated with improved
disease-free and overall survival compared with interferon alfa, which had been
the most promising adjuvant therapy prior to the development of checkpoint
inhibitor immunotherapy. Among previously untreated patients with metastatic
melanoma, nivolumab alone or combined with ipilimumab results in significantly
longer progression-free survival than ipilimumab alone. In patients with PD-L1–
negative tumors, the combination of PD-1 and CTLA-4 blockade appears more
effective than either agent alone (173). The combined inhibition of T-cell
checkpoint pathways by nivolumab and ipilimumab in previously untreated
BRAF wild-type melanoma was associated with a 55% objective response rate
compared with 11% with nivolumab monotherapy (174). For patients whose
tumor contains a BRAF V600 mutation, adjuvant therapy targeting the mitogenactivated protein (MAP) kinase pathway with a combination of a BRAF inhibitor,
such as dabrafenib, and a MEK inhibitor, such as trametinib, is an important
treatment option (175).
Traditionally, melanomas were thought to be radiation resistant. However,
radiation therapy can provide effective palliation in the setting of unresectable,
locally recurrent, or symptomatic metastatic disease. Stereotactic radiosurgery
and stereotactic body radiation therapy can be particularly useful in ablating
oligometastatic disease.
Prognosis
The behavior of melanomas can be unpredictable, but the prognosis is poor.
The reported 5-year overall survival rate for vulvar melanoma is in the
range of 50% to 60% (153,154,164). A SEER database analysis showed diseasespecific survival rates for patients with localized, regional, and distant disease of
76%, 39%, and 22%, respectively (164). Localized disease, negative lymph
nodes, and younger age were significant independent prognostic factors for
improved survival. Because vulvar melanoma has a propensity for late
recurrences, 5-year survival may not reflect cure. Prognosis is best predicted by
microstaging. Patients with lesions invading to 1 mm or less have a good
prognosis, but as depth of invasion increases, the prognosis worsens. For the
primary tumor (T), increasing tumor thickness, an increased mitotic rate, and the
presence of ulceration (i.e., the loss of the epidermal layer overlying the primary
tumor) are associated with an increased risk of relapse. The AJCC stage correlates
well with prognosis and takes into consideration tumor thickness, ulceration,
lymph node status, in-transit, satellite, and/or microsatellite metastases, distant
2733metastases, and serum Lactate dehydrogenase (LDH) level. Additional prognostic
factors are the patient’s age, central tumor location, histologic growth pattern,
lymph–vascular space involvement, aneuploidy, and elevated serum S-100B
protein (153,163,170,171,176–181).
BARTHOLIN GLAND CARCINOMA
Epidemiology
Primary carcinoma of the Bartholin gland is a rare form of vulvar cancer,
which accounts for about 2% to 7% of vulvar malignancies (182). Because of
its rarity, individual experience with the tumor is limited, and recommendations
for management must be based on the review of small published series (43,182–
184). Bartholin gland carcinoma is five times more common in postmenopausal
than in premenopausal women (185).
Histopathology
The bilateral Bartholin glands are greater vestibular glands situated
posterolaterally in the vulva. Their main duct is lined with stratified squamous
epithelium, which changes to transitional epithelium as the terminal ducts are
reached. Because tumors may arise from the gland or the duct, a variety of
histologic types occur, including adenocarcinomas, squamous carcinomas, and,
rarely, transitional cell, adenosquamous, and adenoid cystic carcinomas. A series
from the MD Anderson Cancer Center reported squamous histology in 88% of the
cases (184).
Classification of a vulvar tumor as a Bartholin gland carcinoma typically
required that it fulfill Honan’s criteria, which are as follows:
1. The tumor is in the correct anatomic position.
2. The tumor is located deep in the labium majus.
3. The overlying skin is intact.
4. There is some recognizable normal gland present.
Strict adherence to these criteria results in underdiagnosis of some cases. Large
tumors may ulcerate through the overlying skin and obliterate the residual normal
gland. Although transition between normal and malignant tissue is the best
criterion, some cases will be diagnosed on the basis of their histologic
characteristics and anatomic location.
Signs and Symptoms
2734The most common initial symptom of Bartholin gland carcinoma is a vulvar
mass or perineal pain. About 10% of patients have a history of inflammation of
the Bartholin gland, and malignancies may be mistaken for benign cysts or
abscesses. Delay of diagnosis is common, particularly for premenopausal patients.
A biopsy with histologic evaluation is indicated for all cases with a palpable or
visible solid mass within a Bartholin cyst or abscess, and in all instances where a
presumed Bartholin cyst or abscess does not respond to treatment.
Treatment
Traditionally, treatment was radical vulvectomy with bilateral groin and pelvic
node dissection (186). There seems to be no indication for dissection of the pelvic
nodes in the absence of positive groin nodes, and good results are reported with
hemivulvectomy or radical local excision for the primary tumor (183,184).
Because these lesions are deep in the vulva, extensive dissection is required in the
ischiorectal fossa; surgical margins are often close. Patients with Bartholin gland
carcinoma are more likely to require postoperative radiation therapy (184).
Postoperative radiation to the vulva decreased the likelihood of local recurrence
from 27% (6 of 22 patients) to 7% (1 of 14 patients) (183). If the ipsilateral groin
nodes are positive, bilateral groin and pelvic irradiation may decrease regional
recurrence. If the tumor is fixed to the inferior pubic ramus or involves adjacent
structures, such as the anal sphincter or rectum, preoperative radiation and
chemotherapy is preferable to avoid ultra-radical surgery. A report of 10
consecutive patients with primary Bartholin gland carcinoma suggests that
treatment with radiation or chemoradiation using teletherapy combined with a
boost to the primary site or regional nodes and/or interstitial brachytherapy may
offer an effective alternative to surgery with 3- and 5-year survival rates of 72%
and 66%, respectively (187).
Prognosis
Because of the deep location of the gland, disease tends to be more advanced
than squamous carcinomas at the time of diagnosis but, stage for stage, the
prognosis is similar. A recent single-institution series from the MD Anderson
Cancer Center reported on 429 patients with invasive vulvar carcinoma (184) and
compared 33 patients with Bartholin gland carcinoma with 396 patients with nonBartholin carcinoma. Patients with Bartholin gland carcinoma had a younger
median age (57 vs. 63 years), higher rate of stage III/IV disease (61% vs. 36%),
and were more likely to receive radiation therapy (79% vs. 44%). However, there
was no significant difference in recurrence-free and overall survival. Five-year
disease-free survival rates by stage are summarized in Table 40-8.
2735ADENOID CYSTIC CARCINOMA OF THE BARTHOLIN GLAND
The adenoid cystic variety accounts for 15% of Bartholin gland carcinomas.
A review of 62 cases reported in the literature demonstrates that adenoid
cystic carcinoma of the Bartholin gland is a slow-growing tumor
characterized by perineural infiltration and a marked propensity for local
relapse preceding distant recurrences by years. It is less likely to metastasize
to lymph nodes and carries a somewhat better prognosis (Fig. 40-8) (188–
190). The slowly progressive nature of these tumors and the tendency for late
recurrences are reflected in the disparity between progression-free interval and
overall survival (190).
OTHER ADENOCARCINOMAS
Adenocarcinomas of the vulva usually arise in a Bartholin gland or occur in
association with Paget disease. They may arise rarely from the skin appendages,
paraurethral glands, minor vestibular glands, aberrant breast tissue, endometriosis,
or a misplaced cloacal remnant (191).
Adenosquamous Carcinoma
A particularly aggressive type of carcinoma is the adenosquamous
carcinoma. This tumor has a number of synonyms, including cylindroma,
pseudoglandular squamous cell carcinoma, adenoid squamous cell carcinoma, and
adenoacanthoma of the sweat gland of Lever. The tumor has a propensity for
perineural invasion, early lymph node metastasis, and local recurrence. One study
noted a crude 5-year survival rate of 5.5% (1 of 18) for adenosquamous
carcinoma of the vulva, compared with 62.3% (48 of 77) for patients with
squamous cell carcinoma (192). Treatment should be radical surgical resection
and groin dissection. Postoperative radiation therapy may be appropriate.
Table 40-8 Survival of Patients With Bartholin Gland Carcinoma
FIGO
Stage
No. of
Patients
No. of Patients With
Recurrent Disease
No. of Patients NED at
Last F/Ua
I 15 (21%) 3 (20%) 14 (93%)
II 16 (23%) 2 (13%) 15 (94%)
III 30 (42%) 11 (37%) 22 (73%)
IV 10 (14%) 5 (50%) 5 (50%)
2736Total 71 (100%) 21 (30%)b 56 (79%)b
From references (43,185,186,188).
aMedian follow-up in each study was at least 5 years.
b
Total >100% because some patients with recurrence remained NED.
FIGO, International Federation of Gynecology and Obstetrics; NED, no evidence of
disease; F/U, follow-up.
Basal Cell Carcinoma
Basal cell carcinomas represent about 2% of vulvar cancers. As with other
basal cell carcinomas, vulvar lesions commonly appear as a “rodent ulcer” with
rolled edges, although nodules and macules are other morphologic varieties that
occur. Most lesions are smaller than 2 cm in diameter and are usually situated on
the anterior labia majora. Giant lesions occasionally occur (193). Basal cell
carcinoma usually affects postmenopausal white women and is locally aggressive.
Symptoms are frequently present for a prolonged period and most often include
pruritus, soreness, and irritation (194). It is diagnosed by biopsy, and radical
local excision is adequate treatment (195). Metastasis to regional lymph
nodes is reported but is rare (196–198). The local recurrence rate is about 10%
to 20% (194,199). Basal cell carcinoma of the vulva is associated with a high
incidence of antecedent or concomitant malignancies elsewhere (194). In a series
of 28 women with vulvar basal cell carcinoma, 10 patients had other basal cell
carcinomas, and 10 patients suffered from other primary malignancies (194).
FIGURE 40-8 Adenoid cystic tumor of the Bartholin gland. Basaloid cells form
cribriform, sieve-like spaces containing mucinous material. The hyaline stroma is another
distinct feature of this tumor.
About 3% to 5% of basal cell carcinomas contain a malignant squamous
component, the so-called basosquamous carcinoma. These lesions are more
aggressive and should be treated as squamous carcinomas (198). Another subtype
of basal cell carcinoma is the adenoid basal cell carcinoma, which must be
differentiated from the more aggressive adenoid cystic carcinoma arising in a
Bartholin gland or the skin (198).
Verrucous Carcinoma
Verrucous carcinoma is a variant of squamous cell carcinoma and has
distinctive clinical and pathologic characteristics (200). Although most
commonly found in the oral cavity, verrucous lesions may be found on any moist
membrane composed of squamous epithelium (201). In the female genital tract,
these lesions may develop on the cervix, vulva, and vagina. The cause of the
lesion in the female genital tract is not fully understood, but associations with
2738HPV-6 and HPV-11 were found in some studies, whereas others found no
association with HPV infection (202,203). Some studies found as many as onethird of the cases to have coexisting squamous carcinoma of the vulva,
underscoring the importance of careful histopathologic assessment of these
tumors (204).
Grossly, the tumors have a cauliflower-like appearance; microscopically, they
contain multiple papillary fronds that lack the central connective tissue core that
characterizes condylomata acuminata (Fig. 40-9). The gross and microscopic
features of a verrucous carcinoma are very similar to those of the giant
condyloma of Buschke-Loewenstein, and they probably represent the same
disease entity (191). Adequate biopsy from the base of the lesion is required to
differentiate a verrucous carcinoma from a benign condyloma acuminatum or a
squamous cell carcinoma with a verrucous growth pattern.
FIGURE 40-9 Verrucous carcinoma of the vulva. Note the exophytic hyperkeratotic
papillary fronds and endophytic bulky rete pegs with smooth borders.
Verrucous carcinomas usually occur in postmenopausal women, and they
are slow-growing but locally destructive lesions. Even bone may be invaded.
Metastasis to regional lymph nodes is rare but was reported (205). Radical local
excision is the basic treatment, although any palpably suspicious groin nodes
should be evaluated with fine-needle aspiration cytology or excisional biopsy.
Usually, enlarged nodes will be caused by inflammatory hypertrophy (206). If the
nodes contain metastases, radical vulvectomy and bilateral inguinal–femoral
lymphadenectomy are indicated.
Several small studies failed to document any therapeutic advantage with
radiation therapy (206). There is concern that radiation may induce anaplastic
2740transformation with subsequent regional and distant metastasis (207). One study
reported a corrected 5-year survival rate of 94% for 17 patients treated with
surgery alone, compared with 42% for 7 patients treated with surgery and
radiation (206). The latter patients had more advanced disease. If there is a
recurrence, further surgical excision is the treatment of choice, which
occasionally may necessitate some type of exenteration.
VULVAR SARCOMA
Sarcomas represent 1.5% of vulvar malignancies and constitute a
heterogeneous group of tumors (208). Leiomyosarcomas are the most common,
and other histologic types include fibrosarcomas, neurofibrosarcomas,
liposarcomas, rhabdomyosarcomas, angiosarcomas, epithelioid sarcomas, and
malignant schwannomas.
Leiomyosarcomas usually appear as enlarging, often painful masses, in the
labium majus. Smooth muscle tumors of the vulva that show at least three of the
following four criteria should be regarded as sarcomas: (i) diameter greater than 5
cm, (ii) infiltrating margins, (iii) 5 or more mitotic figures per 10 high-power
fields, (iv) moderate-to-severe cytologic atypia (209). The absence of one, or even
all, of these features does not guarantee against recurrence (210). Lymphatic
metastases are uncommon, and radical local excision is the usual treatment.
Epithelioid sarcomas characteristically develop in the soft tissues of the
extremities of young adults but rarely may occur on the vulva. A description
of two cases and review of three other reports concluded that these tumors might
mimic a Bartholin cyst, leading to inadequate initial treatment (211). The study
indicated that vulvar epithelioid sarcomas behave more aggressively than their
extragenital counterparts, with four of the five patients dying of metastatic
disease, and suggested that early recognition and wide excision should improve
the prognosis.
Rhabdomyosarcomas are the most common soft tissue sarcomas in
childhood, and 20% involve the pelvis or genitourinary tract (212). Dramatic
gains were made in the treatment of these tumors during the past 20 years.
Previously, radical pelvic surgery was the standard approach, but results were
poor. A multimodal approach evolved, and survival rates improved significantly,
with a corresponding decrease in morbidity. In a report of the experience of the
Intergroup Rhabdomyosarcoma Study I and II (1972 to 1984) with primary
tumors of the female genital tract, nine patients aged 1 to 19 years had primary
vulvar tumors, and these tumors were often regarded as a form of Bartholin gland
infection before biopsy (213). They were all managed with chemotherapy
(vincristine, or actinomycin D and cyclophosphamide and doxorubicin), with or
2741without radiotherapy. Wide local excision of the tumor, with or without inguinal–
femoral lymphadenectomy, was carried out before or after the chemotherapy.
Seven of the nine patients were free of disease 4 or more years from diagnosis,
one patient was free of disease when lost to follow-up at 5 years, and one patient
was alive with disease.
RARE VULVAR MALIGNANCIES
In addition to the previously mentioned tumors, a number of malignancies more
commonly seen in other areas of the body may rarely occur as isolated vulvar
tumors.
Lymphomas
The genital tract may be involved primarily by malignant lymphomas but,
more commonly, involvement is a manifestation of systemic disease. In the
lower genital tract, the cervix is most often involved, followed by the vulva and
the vagina (214). Most patients are in their third to sixth decades of life, and about
three-fourths of the cases involve diffuse large cell or histiocytic non-Hodgkin
lymphomas. The remainder are nodular or Burkitt lymphomas. Treatment is by
surgical excision followed by chemotherapy and radiation or both, and the overall
5-year survival rate is approximately 70% (214).
Endodermal Sinus Tumor
There were four case reports of endodermal sinus tumor of the vulva, and three of
the four patients died of distant metastases (215). All patients were in their third
decade of life, but none was treated with modern chemotherapy.
Merkel Cell Carcinoma
Merkel cell carcinomas are primary small cell carcinomas of the skin that
resemble oat cell carcinomas of the lung. They metastasize widely and have a
very poor prognosis (216–218). They should be locally excised and treated with
cisplatin-based chemotherapy.
Dermatofibrosarcoma Protuberans
This rare, low-grade cutaneous malignancy occasionally involves the vulva. It has
a marked tendency for local recurrence but a low risk of systemic spread (219).
Radical local excision should be sufficient treatment.
2742METASTATIC TUMORS OF THE VULVA
Metastatic tumors to the vulvas are rare. The most common primary site is the
cervix, followed by the endometrium, kidney, and urethra. Most patients in whom
vulvar metastases develop have advanced primary tumors when diagnosed, and in
approximately one-fourth of the patients, the primary lesion and the vulvar
metastasis are diagnosed simultaneously (220)



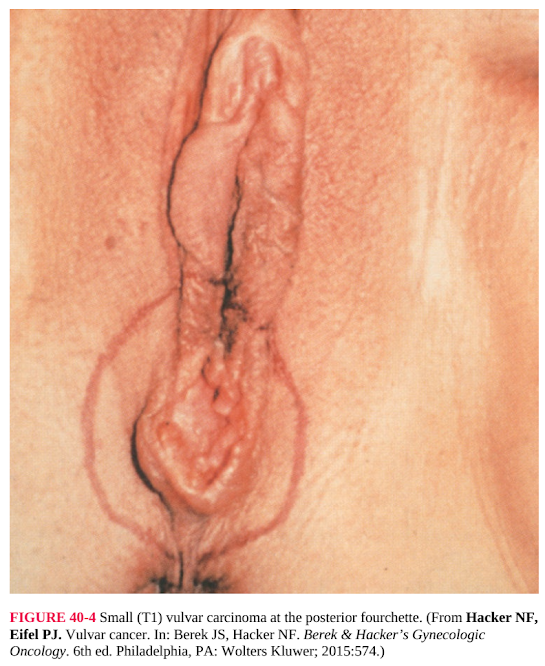





Nhận xét
Đăng nhận xét