Chapter 11. First and Second Trimester Pregnancy Loss
BS. Nguyễn Hồng Anh
Miscarriage is a common event in pregnancy. Most early losses stem rom genetic abnormalities, and thus the opportunity or prevention is small. Women with later miscarriage or with recurrent miscarriage more likely have a chronic etiology that may be modied. In contrast to these spontaneous losses, pregnancy termination may be elected. For both induced abortion and miscarriage, management options include surgery or medication, and providers should have an understanding o these methods and their potential complications.
NOMENCLATURE
Abortion is dened as the spontaneous or induced termination o pregnancy beore etal viability. Many preer miscarriage or spontaneous loss. Induced abortion describes termination with surgery or medication o a live etus that has not reached viability. Denitions o an abortus vary among organizations. Te National Center or Health Statistics and the World Health Organization dene abortion as loss or termination o a pregnancy with a etus aged younger than 20 weeks’ gestation or weighing <500 g. Tese criteria, however, are somewhat contradictory because the mean birthweight o a 20-week etus approximates 330 g, whereas 500 g is the mean or 22 weeks (Hadlock, 1991). Further conusion may derive rom criteria that are set by state laws and dene abortion even more widely.
Incongruity also exists or the term early pregnancy loss itsel. Te American College o Obstetricians and Gynecologists (2019b) denes this as a nonviable, intrauterine pregnancy (IUP) within the rst 126/7 weeks o gestation that consists o either an empty gestational sac or one containing an embryo or etus without etal heart activity. Recurrent pregnancy loss is variably dened but is meant to identiy women with repetitive miscarriage (p. 203). Other denitions help distinguish intrauterine rom ectopic gestations. Te term pregnancy of unknown location (PUL) describes a pregnancy identied by human chorionic gonadotropin (hCG) level testing but without a conrmed sonographic location. In this context, ve categories are proposed or early pregnancies: denite ectopic pregnancy, probable ectopic pregnancy, PUL, probable IUP, and denite IUP (Barnhart, 2011).
Diagnostic and management options or ectopic gestation are described in Chapter 12. Last, spontaneous abortion includes subcategories o threatened, incomplete, complete, missed, and inevitable abortions. Tese are discussed in the next sections. Septic abortion is used to urther classiy any o these that are complicated by inection.
FIRST-TRIMESTER SPONTANEOUS ABORTION
■ Pathogenesis
More than 80 percent o spontaneous abortions occur within the rst 12 weeks o gestation. With rst-trimester losses, demise o the embryo or etus nearly always precedes spontaneous expulsion. Death is usually accompanied by hemorrhage into the decidua basalis. Tis is ollowed by adjacent tissue necrosis that stimulates uterine contractions and expulsion. An intact gestational sac is usually lled with uid. Anembryonic miscarriage or preembryonic loss describes the group with no identiable embryo. Te term blighted ovum is less preerred. An embryonic miscarriage or embryonic loss displays an embryo without cardiac activity during ultrasound evaluation (Kolte, 2015; Pinar, 2018).
In second-trimester losses, the etus usually does not die beore expulsion. Tus, other sources or abortion are sought and described on page 213.
■ Incidence
Rates or miscarriage vary according to the study population. In pregnancies aged 5 to 20 weeks’ gestation, the incidence ranges rom 10 to 20 percent and is higher in earlier weeks (Ammon Avalos, 2012). o evaluate rates starting at conception, researchers have tested daily urinary samples or β-hCG in women trying to conceive and ound that approximately 20 percent o pregnancies ailed very early (Wang, 2003). Tese biochemical pregnancy losses are clinically silent and are identi- ed only by dropping β-hCG levels (Kolte, 2015). Certain actors inuence the clinically apparent miscarriage rate and are described next. It is unknown i these same actors also aect biochemical pregnancy loss.
■ Fetal Factors
O all miscarriages, approximately hal are euploid abortions, that is, carrying a normal chromosomal complement. Te other hal o all miscarriages has a chromosomal abnormality. Tis percentage appears to persist even rom evaluations with newer cytogenetic techniques (Sahoo, 2017). However, outside o research, routine use o chromosomal microarray testing o rst-trimester etal tissues is not endorsed by the American College o Obstetricians and Gynecologists (2020d). Te American Society or Reproductive Medicine (2012) recognize its value only i cytogenetic analysis alters uture care o a patient.
Both abortion and chromosomal anomaly rates decline with advancing gestational age (Eiben, 1990). O chromosomally abnormal embryos, 75 percent aborts by 8 weeks’ gestation. Te rate o abortion with euploid etuses peaks at approximately 13 weeks (Kajii, 1980). O chromosomal abnormalities, 95 percent are caused by maternal gametogenesis errors, and 5 percent by paternal errors (Jacobs, 1980). Tus, the aneuploid abortion incidence rises dramatically ater maternal age exceeds 35 years (Eiben, 1990).
Indeed, maternal age is a primary actor underlying the spontaneous loss o aneuploid etuses (Nybo Andersen, 2000). o a lesser degree, increasing paternal age is also associated with a greater abortion risk (Nguyen, 2019). Not yet well studied, chromosomal abnormalities in spermatozoa likely play a role (Pohl, 2021).
Most common abnormalities are trisomy, ound in 50 to 60 percent; monosomy X, in 9 to 13 percent; and triploidy, in 11 to 12 percent (Jenderny, 2014; Sahoo, 2017). risomies typically result rom isolated nondisjunction, and rates rise with maternal age (Boué, 1975). risomies o chromosomes 13, 16, 18, 21, and 22 are most common. Less oten, a trisomy orms rom a balanced structural chromosomal rearrangements. Tese may originate rom either parent and are ound in 2 to 4 percent o couples with recurrent pregnancy loss (p. 204). Monosomy X (45,X) is the single most requent specic chromosomal abnormality. Tis is urner syndrome, which usually results in miscarriage, but liveborn emales are described in Chapter 3 (p. 36). Conversely, autosomal monosomy is rare and incompatible with lie.
riploidy is an additional haploid set o chromosomes, and cells thus contain a total o 69 chromosomes. Te extra haploid set can be either maternally or paternally derived. Maternally derived digynic triploidy typically results rom a meiosis error and ertilization o a diploid ovum by a normal haploid sperm. Paternally derived diandric triploidy usually orms rom the ertilization o a normal haploid ovum by two haploid spermatozoa and leads to partial molar pregnancy (Chap. 13, p. 237). riploid etuses requently spontaneously abort early, and the ew carried longer are all grossly deormed.
■ Maternal Factors
Medical Disorders
In chromosomally normal pregnancy losses, maternal contributions can play a role. For example, a prominent miscarriage risk is associated with poorly controlled diabetes mellitus, obesity, thyroid disease, and systemic lupus erythematosus. In these, inammatory mediators may be an underlying theme to pregnancy loss (Kalagiri, 2016; Sjaarda, 2017). In chapters on these disorders, the miscarriage rate and attempts to lower it are discussed. In contrast, thrombophilias are no longer linked to miscarriage (American College o Obstetricians and Gynecologists (2020b). For women undergoing cancer treatment, direct therapeutic radiation can cause miscarriage. Suggested sae parameters are ound in Chapter 49 (p. 872). Similarly, the eects o chemotherapy on miscarriage rates are not well dened. Particularly worrisome are women with an ongoing pregnancy ater early exposure to the teratogen methotrexate, described in Chapter 8 (p. 152). Cancer survivors previously treated with abdominopelvic radiotherapy and now subsequently pregnant may carry a greater risk or miscarriage (Chap. 66, p. 1164).
Surgical Procedures
Te miscarriage risk associated with surgery is not well studied. But, as discussed in Chapter 49 (p. 867), uncomplicated surgical procedures perormed during early pregnancy are unlikely to raise this risk (Mazze, 1989). I indicated, ovarian tumors can generally be resected without inciting miscarriage. An exception involves early removal o the corpus luteum or the entire ovary in which it resides. I perormed beore 10 weeks’ gestation, supplemental progesterone should be given and is described in Chapter 66 (p. 1170). rauma seldom causes rst-trimester miscarriage, and although Parkland Hospital is a busy trauma center, this is an inrequent association. Major trauma—especially abdominal— can cause etal loss but is more likely as pregnancy advances (Chap. 50, p. 891).
Nutrition
Sole deciency o one nutrient or moderate deciency o all does not appear to raise miscarriage risks. Even in extreme cases—or example, hyperemesis gravidarum—abortion is rare. Dietary quality may play a small role, and some data suggest miscarriage risk may decline in women who eat a diet rich in ruits, vegetables, whole grains, and sh (Gaskins, 2015). Unlike obesity, underweight is not associated with a greater miscarriage risk (Balsells, 2016).
With caeine, reports link heavy intake o approximately ve cups o coee per day—about 500 mg o caeine—with a slightly greater abortion risk (Cnattingius, 2000; Klebano, 1999). Values vary depending on brewing style, but an 8-ounce cup o coee contains 80 to 100 mg o caeine. Black or green tea has hal this dose (Food and Drug Administration, 2018).
Currently, the American College o Obstetricians and Gynecologists (2020e) concludes consumption o <200 mg/d likely is not a major miscarriage risk and that any associated risk with higher intake is unsettled. Metaanalyses support an increasing dose-related risk (Chen, 2016; Li, 2015).
Behavioral Factors
Approximately 7 percent o pregnant women acknowledge cigarette smoking (Kondracki, 2019). One metaanalysis ound a slight dose-related relationship between current smoking and early pregnancy loss (Pineles, 2014). Te serious later risks o persistent smoking on pregnancy outcomes are discussed in Chapter 8 (p. 156). Alcohol consumption carries miscarriage risk mainly in those with chronic or heavy use (Feodor Nilsson, 2014). Te potent teratogenic eects in these instances are also discussed in Chapter 8 (p. 149).
Environmental Factors
Despite the many inections acquired in pregnancy, these uncommonly cause early miscarriage. Te important maternoetal consequences o specic inections in later pregnancy are discussed in Chapters 67 and 68. Environmental toxins suggested to have a possible link to miscarriage include bisphenol A, phthalates, polychlorinated biphenyls, and dichlorodiphenyltrichloroethane (DD) (Krieg, 2016). Even ewer studies implicate occupational exposures. Data suggest a slight increased miscarriage risk in health-care workers exposed to radiation or antineoplastic drugs (Anderson, 2020). Te National Institute or Occupational Saety and Health publishes guidelines on potentially hazardous drugs (Connor, 2016). Evidence implicating occupational anesthetic gases in miscarriage is not robust (Oliveira, 2021). Still, gas scavenging systems and workplace exposure limits are recommended (McGlothin, 2014).
■ Spontaneous Abortion Clinical Classification
Threatened Abortion
Tis is dened as bleeding through a closed cervical os in the rst 20 weeks o pregnancy and with a live embryo or etus. It may portend abortion, or it may be associated with conceptus implantation. Other bleeding sources to exclude are ectopic pregnancy, cervical inection, and dysplastic or neoplastic cervical lesions. Almost one ourth o women develop bleeding during early gestation that is attributed to threatened abortion (Everett, 1997). Bleeding or spotting may persist or days or weeks. It may be accompanied by suprapubic discomort, mild cramps, pelvic pressure, or persistent low backache. O symptoms, bleeding is by ar the most predictive actor or subsequent pregnancy loss.
Vaginal bleeding or abdominal pain in early pregnancy should prompt hematocrit and blood type assessment. One primary goal is to exclude ectopic pregnancy, and strategic use o β-hCG levels and transvaginal sonography (VS) is outlined in Chapter 12 (p. 222). A second goal is to determine IUP viability. With an IUP, the gestational sac—an anechoic uid collection that represents the exocoelomic cavity—may be seen by 4.5 weeks (Fig. 10-2, p. 177). At this time, β-hCG levels generally measure 1500 to 2000 mIU/mL (Barnhart, 1994; imor-ritsch, 1988). Connolly and colleagues (2013), however, noted that a threshold as high as 3500 mIU/mL may be needed to identiy the gestational sac in some cases that ultimately yield a viable singleton IUP. One caveat during VS is that a gestational sac may appear similar to a pseudogestational sac, which is an anechoic intrauterine uid collection (Fig. 12-3, p. 223). Tis pseudosac may be blood derived rom a bleeding ectopic pregnancy and is easier to exclude once a yolk sac is seen. ypically, the yolk sac is visible by 5.5 weeks and with a mean gestational-sac diameter o 10 mm. Tus, the diagnosis o an IUP should be made cautiously i the yolk sac is not yet seen (American College o Obstetricians and Gynecologists, 2020h). Fetal cardiac activity can typically be detected at 6 to 6.5 weeks.
A subchorionic hematoma may also be seen sonographically with threatened miscarriage (Fig. 11-1). In general, this collection does not portend a greater miscarriage risk (Naert, 2019). Once threatened abortion is diagnosed, observation is the norm. Acetaminophen-based analgesia will help relieve
FIGURE 11-1 In this transvaginal sonogram sagittal view of the uterus, a subchorionic hematoma is seen as a very hypoechoic collection (arrow). It lies adjacent to the round anechoic gestational sac that contains a nearly 7-week embryo (calipers). (Reproduced with permission from Jason McWhirt, ARDMS.)
cramping discomort. Te randomized Progesterone in Spontaneous Miscarriage (PRISM) trial ound no advantage to progesterone supplementation to lower miscarriage rates in women with rst-trimester bleeding (Coomarasamy, 2019). Bed rest does not improve outcomes and may cause harms such a deepvein thrombosis (McCall, 2013). We do counsel against intercourse until bleeding subsides. In unusual cases, bleeding with threatened abortion can lead acute severe anemia or hypovolemia. Pregnancy evacuation is generally indicated. Less oten, transusion and urther observation is elected.
Even i miscarriage does not ollow threatened abortion, later rates o preterm birth and placental abruption are slightly increased (Saraswat, 2010). Weiss and coworkers (2004) noted greater risks or later adverse outcomes i early bleeding was heavy rather than light. Despite these associations, we typically do not add sonography or other surveillance later in pregnancy solely or a rst-trimester diagnosis o threatened abortion.
Incomplete Abortion
During miscarriage, the cervix opens and placental separation causes bleeding. Beore 10 weeks’ gestation, the etus and the placenta are requently expelled together, but later, they oten deliver separately. Tus, tissue may remain entirely within the uterus or partially extrude through the cervix. Products lying loosely within the cervical canal can be easily extracted or teased out with ring orceps. For uterine inection or or hemodynamically unstable women with heavy bleeding, prompt surgical evacuation is perormed.
For less urgent cases, three management options are curettage, expectant management, or misoprostol (Cytotec), which is prostaglandin E1 (PGE1) (Kim, 2017). With all three, treatment complications such as inection and need or transusion are inrequent. However, misoprostol and expectant care can be associated with unpredictable bleeding. Tus, some women will still require unscheduled curettage, which studies have used as a ailure endpoint. Expectant management o spontaneous incomplete abortion has ailure rates that approximate 25 percent in randomized trials (Nielsen, 1999; rinder, 2006). Medication therapy carries ailure rates o 5 to 30 percent (Shochet, 2012; rinder, 2006).
Many o the studies assessing treatment have used an 800-μg vaginal, a 400-μg sublingual, or a 600-μg oral misoprostol dose. Last, curettage usually results in a quick resolution that is 95- to 100-percent successul. However, it is invasive, carries surgical risks, and is not necessary or all women (p. 211).
Complete Abortion
At times, the entire pregnancy is expelled. With completion, bleeding ebbs, and the internal cervical os subsequently closes over the next hour or so. Patients are encouraged to bring in passed tissue, and a gestation should be documented within blood clots or within a decidual cast (Fig. 11-2). Te latter is the thickened endometrium, in the shape o the uterine cavity, that may slough with miscarriage. I a gestational sac is not identied within the expelled tissue, VS helps dierentiate a complete abortion rom a threatened abortion or ectopic pregnancy. Findings o complete abortion include a thin endometrium without a gestational sac. However, this does not guarantee a recent IUP. One study evaluated 152 women with heavy bleeding and an empty uterus with endometrial thickness <15 mm. Six percent were subsequently ound to have an ectopic pregnancy (Condous, 2005). Tus, a complete abortion cannot be surely diagnosed unless: (1) true products o conception are seen grossly or (2) sonography documents rst an IUP and then later an empty cavity. In unclear settings, serial serum β-hCG levels aid clarication. With complete abortion, these levels drop quickly (Table 11-1).
Missed Abortion
Tis describes dead products o conception that have been retained or days or weeks within a uterus with a closed cervical
FIGURE 11-2 A. Decidual cast from a complete abortion. B. Opening the cast reveals a clear translucent gestational sac, which confirms the diagnosis.
TABLE 11-1. Percentage Decline of Initial Serum β-hCG
aThe percentage decline is given as the expected decline. The minimum expected decline in parentheses is the 95th percentile value. Declines less than this minimum may reflect retained either intrauterine or extrauterine trophoblast. hCG = human chorionic gonadotropin. Data from Barnhart, 2004; Chung, 2006.
os. Diagnosis is imperative prior to intervention and avoids interruption o a potentially live IUP. Again, VS and β-hCG levels are primary tools. With the latter, levels that plateau or drop are typical.
With VS, a 1- to 2-mm embryo adjacent to the yolk sac can be seen at 5 to 6 weeks’ gestation. As listed in Table 11-2, absence o an embryo in a sac with a mean sac diameter (MSD) ≥25 mm signies pregnancy loss. Te MSD is calculated by summing the length, width, and height o a gestational sac and dividing the sum by three. Fetal cardiac activity can typically be detected at 6 to 6.5 weeks, with a crown-rump length (CRL) o 1 to 5 mm, and an MSD o 13 to 18 mm (Goldstein, 1992; Levi, 1990). A CRL threshold ≥7 mm plus absent cardiac activity also is used to diagnose nonviability (Doubilet, 2013). For cases in which a gestational sac has no embryo or yolk sac, additional time and repeat VS are recommended. M-mode should be used to document cardiac activity and measure the rate (Brown, 2018). Pulsed Doppler is not used routinely and is reserved or specic diagnostic purposes because o theoretical temperature elevation in exposed etal tissues (Chap. 14, p. 247).
Less robust sonographic markers may portend pregnancy ailure. A yolk sac diameter >7 mm in pregnancies <10 weeks’ gestation is one (Rodgers, 2015). Te etal heart rate in the rst trimester rises rom 110 to 130 beats per minute (bpm) at 6 weeks’ gestation to 160 to 170 bpm at 8 weeks (Achiron, 1991; Rauch, 2009). A slower heart rate is unavorable, especially one <85 bpm (DeVilbiss, 2020). Even with cardiac activity, etuses with a small MSD may presage embryonic loss. A dierence <5 mm between the MSD and CRL values raises concern (Bromley, 1991). Last, an irregular gestational sac contour may portend loss (Nyberg, 1986).
With embryonic or etal death conrmed, expectant observation, surgery, or medication is an option. Nonsurgical options balance less invasiveness against heavier associated bleeding, longer completion times, and lower success rates. O options, expectant care underperorms medication or surgery, and ailure rates range rom 15 to 50 percent (Luise, 2002; rinder, 2006; Zhang, 2005). Also, weeks may pass between pregnancy ailure diagnosis and actual spontaneous miscarriage.
As a medication option, misoprostol can be given, and a single 800-μg dose vaginally is a common standard. It may be repeated once in 1 to 2 days, and one large trial reported that 22 percent o women required a second dose (Zhang, 2005). Overall, ailure rates range rom 15 to 40 percent (Petersen, 2013; rinder, 2006). Success rates are improved by a 200-mg oral dose o miepristone (Mieprex) given 24 hours prior to misoprostol (Schreiber, 2018). In the United States, access to this antiprogestin is limited by the Food and Drug Administration (FDA) to providers participating in the manuacturer’s
Risk Evaluation and Mitigation Strategy (REMS) (Danco Laboratories, 2021). Contraindications mirror those listed in the section describing induced abortion (p. 212). Inevitable Abortion of a Previable Fetus Either rupture o placental membranes or marked ballooning o membranes into the vagina may lead to inevitable abortion. Te latter is discussed in the cervical insufciency section (p. 205).
Preterm prelabor rupture o membranes (PPROM) at a previable gestational age complicates 0.5 percent o pregnancies (Hunter, 2012). Instead, periviable birth is dened as delivery between 200/7 and 256/7 weeks and is discussed in Chapter 45 (p. 785). Clinically with PPROM, abundant vaginal uid that pools during sterile speculum examination conrms the diagnosis. Patient coughing or Valsalva maneuver may accentuate this. Other diagnostic steps can include pH testing, microscopic examination, and sonographic assessment o amnionic uid volume, and these are outlined in Chapter 22 (p. 426).
Rupture may be spontaneous, and risks are prior PPROM, prior second-trimester delivery, and tobacco use (Kilpatrick, 2006). Iatrogenic uid leakage can ollow amniocentesis or etal surgery. In some second-trimester cases, uid may have collected previously between the amnion and chorion and reects only chorion leakage.
Spontaneous rupture in the rst trimester is nearly always ollowed by either uterine contractions or inection, and termination is typical. With second-trimester spontaneous PPROM at a previable age, 40 to 50 percent o women will deliver within the rst week, and 70 to 80 percent will do so ater 2 to 5 weeks (American College o Obstetricians and Gynecologists, 2020).
Average latency is 2 weeks (Hunter, 2012; Kibel, 2016). Signicant maternal complications attend previable PPROM and include uterine inection, sepsis, placental abruption, postpartum hemorrhage, and retained placenta (Dotters-Katz, 2017b; Waters, 2009). In one study, 56 percent o women suered one or more o these complications (van der Marel, 2016). With signicant bleeding or ever, the uterus should be evacuated. Surgical evacuation or medication options or this vary by gestational age and surgeon skill. Methods mirror those or second-trimester elective abortion, described later (p. 213). For cases in which previable delivery is inevitable or elected, neonatal consultation aids decision-making and helps orm expectations or the amily. For etuses with a lie-limiting
TABLE 11-2. Guidelines for Early Pregnancy Loss
condition, such as extreme prematurity, perinatal palliative care is a strategy that emphasizes comort (American College o Obstetricians and Gynecologists, 2019c). Care team members can include obstetric and neonatal proessionals, chaplaincy, and mental health specialists.
In cases without bleeding or ever, expectant management is an option in the well-counseled patient. Many will choose termination due to the earlier-described maternal risks and tenuous neonatal outcomes. Early etal mortality stems rom pulmonary hypoplasia, severe intraventricular hemorrhage, and sepsis. O those managed expectantly with PPROM at <20 weeks, the percentage o inants who are discharged home ranges rom 12 to 23 percent (Hunter, 2012; Sim, 2020; van der Martel, 2016). Common neonatal morbidities are respiratory distress syndrome, bronchopulmonary hypoplasia or dysplasia, necrotizing enterocolitis, and sepsis. Overall, prognosis is improved i previable PPROM occurs at a later gestation, latency is longer, and oligohydramnios is absent. With the last, poor lung development and etal skeletal deormations can result rom scant amnionic uid.
I expectant care is elected, initial hospital evaluation, diminished physical activity, and observation or ever or labor are reasonable. Ater 24 hours, i there is no bleeding, cramping, or ever, a woman may resume ambulation and is discharged home. She is instructed to watch or ever, contractions, or bleeding. Antibiotics are considered and given or 7 days to extend latency (Dotters-Katz, 2017a). However, lungmaturing corticosteroids, magnesium sulate neuroprophylaxis, group B streptococcus antibiotic prophylaxis, cesarean delivery, and tocolytics are not recommended beore 22 weeks’ gestation (American College o Obstetricians and Gynecologists, 2019d).
Once a viable age is reached, readmission until delivery is our practice. PPROM care at viable gestational ages is described in Chapter 45 (p. 799).
In procedure-related PPROM, iatrogenic leaks are typically higher in the uterus and tend to sel-seal. For leaks rom amniocentesis, management is typically conservative, and brie bedrest yields high pregnancy continuation rates. For rupture ater etal surgery, investigational treatments can address surgical leaks (Chmait, 2017). One is an occlusive plug—termed an amniopatch—that orms ollowing intraamnionic instillation o autologous platelets and cryoprecipitate. Another is amnioinusion to counter the eects o oligohydramnios but is not robustly supported by current data (Roberts, 2014; van Kempen, 2019).
In subsequent pregnancies, the risk or recurrent preterm birth is great in women with prior previable PPROM. In one cohort study, 46 percent delivered their next pregnancy beore 37 weeks, and 17 percent delivered beore reaching 24 weeks (Monson, 2016).
Septic Abortion
With spontaneous or induced abortion, organisms may invade myometrial tissues and extend to cause parametritis, peritonitis, and septicemia. Tis may complicate both medication and surgical methods. Classic clinical ndings include ever, lower abdominal pain, uterine tenderness, and oul vaginal discharge. Vaginal bleeding and sonographic evidence o retained uterine tissue are others. Urinary tract inection should be excluded by urinalysis, and a complete blood count assesses leukocytosis. Most bacteria causing septic abortion are part o the normal vaginal ora. Tus, vaginal culture is not inormative, however, we obtain blood cultures or those with sepsis. Particularly worrisome are severe necrotizing inections and toxic shock syndrome (SS) caused by group A streptococcus—S pyogenes (Dai, 2009). Maternal deaths rom Clostridium perfringens or C sordellii also have been described. With SS, women show severe endothelial injury, capillary leakage, hemoconcentration, hypotension, tachycardia, and marked leukocytosis but may not be ebrile initially.
With septic abortion, management includes prompt administration o broad-spectrum antibiotics as discussed in Chapter 37 (p. 651). I products are retained, suction evacuation is per- ormed. Most women respond to this treatment within 1 to 2 days and are discharged when aebrile. Additional oral antibiotics are likely unnecessary (Savaris, 2011). In a very ew women, severe sepsis syndrome develops, and intensive supportive care is essential. Although rare, widespread peritonitis despite curettage and clinical decline in the patient should raise concerns. Imaging that shows intraabdominal ree air or air within the uterine wall typically prompts laparotomy (Eschenbach, 2015). I the uterus is necrotic, hysterectomy is indicated.
Anti-D Immunoglobulin
With spontaneous or induced abortion, 2 percent o D-negative women will become alloimmunized i not provided passive isoimmunization. With surgical dilation and curettage, this rate may reach 5 percent. Te American College o Obstetricians and Gynecologists (2019e) recommends a 300-μg intramuscular dose o anti-Rho (D) immunoglobulin or all gestational ages. Doses can also be graduated. Namely, a 50-μg or 120-ug dose is given or pregnancies ≤12 weeks and a 300-μg one or those ≥13 weeks. Tis is administered immediately ollowing surgical evacuation. For medication abortion or expectant management, the injection is given within 72 hours o pregnancy ailure diagnosis. With threatened abortion, immunoglobulin prophylaxis is controversial because o sparse evidence-based data (Hannan, 2006). It is reasonable to administer anti-D immunoglobulin or a threatened abortion, and this is our practice.
RECURRENT MISCARRIAGE
Aecting approximately 1 percent o ertile couples, recurrent pregnancy loss (RPL) is classically dened as three or more consecutive pregnancy losses at <20 weeks’ gestation or with a etal weight <500 g. However, data show the risk or a subsequent loss to be similar whether a woman has two or three prior miscarriages (Bhattacharya, 2010). Te American Society or Reproductive Medicine (2020) now denes RPL as two or more ailed pregnancies conrmed by sonographic or histopathological examination. Primary RPL reers to multiple losses in a woman who has never delivered a liveborn, and secondary RPL reers to multiple pregnancy losses in a patient with a prior live birth. Remarkably, chances or a successul pregnancy are >50 percent even ater ve miscarriages (Table 11-3) (Brigham, 1999). RPL evaluation addresses its major causes, described next.
■ Etiology
Widely accepted causes o RPL include some parental chromosomal abnormalities, antiphospholipid antibody syndrome, specic endocrinopathies, and certain structural uterine abnormalities. Tus, current RPL evaluation includes karyotyping o both parents; measuring antiphospholipid antibody levels; assessing HbA1c, thyroid-stimulating hormone (SH), and prolactin levels; and perorming saline-inusion sonography (SIS) or hysterosalpingography (American Society or Reproductive Medicine, 2012). Te timing o recurrent loss can oer clues, and in some women, each miscarriage may occur near the same gestational age (Heuser, 2010). Approximately 50 percent o women have idiopathic RPL (Habayeb, 2004).
■ Parental Chromosomal Abnormalities
In rst-trimester RPL, the incidence o genetic abnormalities is signicantly lower than in sporadic miscarriage (Stephenson, 2002). A notable exception is a parent that carries a reciprocal translocation or Robertsonian translocation (Fan, 2016). Teir genesis and reproductive sequelae are discussed in Chapter 16 (p. 316). Tese account or only 2 to 4 percent o RPL cases, but karyotyping o both parents is a recognized part o RPL evaluation. Ater thorough genetic counseling, couples with an abnormal karyotype can be oered in vitro ertilization (IVF) ollowed by preimplantation genetic testing (PG) or oered donor gametes (Chap. 17, p. 348). However, birth rates with IVF plus PG are not superior to expectant management or this cause o RPL (Murugappan, 2016). Importantly, in couples with RPL who are chromosomally normal, PG is not recommended solely or the indication o RPL.
■ Anatomical Factors
Several genital tract abnormalities have been implicated in RPL, but direct linkage is not robust. According to Devi Wold and associates (2006), 15 percent o women with ≥3 consecutive miscarriages will have a congenital or acquired uterine anomaly. Tus, SIS and hysteroscopy are primary evaluation tools. Uterine leiomyomas are common, and some may cause miscarriage, especially i located near the placental implantation site. However, data suggesting a signicant link to RPL are not convincing and poor quality (Saravelos, 2011). Te American Society or Reproductive Medicine (2017) notes that hysteroscopic excision o large submucosal leiomyomas in women withRPL can be considered. Uterine synechiae, oten called Asherman syndrome, result rom broad destruction o endometrium and subsequent scarring, which can ollow uterine curettage or hysteroscopic surgeries. With SIS, multiple hypoechoic bridging bands are seen to span the endometrial cavity, which is lled with anechoic saline. reatment is hysteroscopic adhesiolysis, but success rates are lower with more severe initial disease.
Uterine polyps have been ound more requently in women with RPL, but causality is unclear. Tese are seen during hysteroscopy or SIS as a mass lesion extending rom the uterine wall into the endometrial cavity. Application o color Doppler during SIS classically reveals a single eeder vessel reaching the mass. Hysteroscopic polypectomy can be considered.
For all these acquired lesions, correction can be considered or those with signicant uterine cavity distortion. However, evidence supporting improved birth rates is not robust (American Society or Reproductive Medicine, 2012). Moreover, reproductive benets are balanced against intrauterine adhesions that may orm ater any intracavitary surgery.
Congenital uterine anomalies oten originate rom abnormal müllerian duct ormation. Depending on their anatomy, some may raise risks or miscarriage or preterm delivery. O these, septate uterus is most closely linked with miscarriage. Hysteroscopic resection has been associated with improved live birth rates in some but not all studies (Rikken, 2017). It can be considered or RPL (American Society or Reproductive Medicine, 2016). Chapter 3 (p. 42) contains a uller discussion o uterine abnormalities and their other obstetrical eects.
■ Immunological Factors
Miscarriages are more common in women with systemic lupus erythematosus (SLE) (Clowse, 2008). Many o these women and also some without SLE carry antiphospholipid antibodies. Tese are a amily o autoantibodies that bind to phospholipidbinding plasma proteins and are associated with RPL (AlijotasReig 2019). As shown in Table 11-4, the antiphospholipid antibody syndrome (APS) is dened by these antibodies in combination with various orms o reproductive loss or vascular thrombosis (American College o Obstetricians and Gynecologists, 2019a). Chapter 62 (p. 1114) describes pregnancy loss with APS and treatment.
■ Endocrine Factors
O recurrent miscarriages, 8 to 12 percent are caused by endocrine actors (Arredondo, 2006). First, the well-known abortiacient action o uncontrolled diabetes mellitus is detailed in Chapter 60 (p. 1070). Optimal periconceptional glycemic control will mitigate many o these losses.
Overt hypothyroidism and severe iodine deciency also raise miscarriage rates (Chap. 61, p. 1094). Correction with supplementation reverses these actions. Subclinical hypothyroidism, however, does not appear to increase miscarriage rates (Dong, 2020). Antithyroid antibodies are a common associate o subclinical hypothyroidism or overt hypothyroidism. A recent
TABLE 11-3. Predicted Success Rate of Subsequent Pregnancy According to Age and Number of Previous Miscarriages
metaanalysis ound positive associations between these antibodies and a greater risk or sporadic and recurrent miscarriages (Xie, 2020). However, levothyroxine supplementation in this group does not improve subsequent pregnancy outcomes (Sun, 2020). For evaluation o RPL, measuring levels o serum SH, but not antithyroid antibodies, is reasonable.
Other endocrinopathies are inconclusively implicated. One is progesterone deciency caused by a luteal-phase deect. Progesterone supplementation, compared with placebo, did not improve the live birth rate in those with RPL in one large trial (Coomarasamy, 2015). Limited data implicate hyperprolactinemia (Hirahara, 1998). Obesity, polycystic ovarian syndrome, and insulin resistance are also linked to RPL (Cavalcante, 2019; Craig, 2002; Mayrhoer, 2020). However, the interplay between these makes assigning individual causality difcult.
SECOND-TRIMESTER ABORTION
■ Etiology
Te timespan that denes a midtrimester etal loss extends rom the end o the rst trimester until the etus weighs <500 g or gestational age reaches 20 weeks. Te spontaneous loss rate in the second trimester is much lower than in the rst. Unlike earlier miscarriages that requently stem rom chromosomal aneuploidies, causes o these later etal losses are more diverse (Table 11-5). Teir ultimate presentations may be miscarriage, PPROM, or etal demise prior to labor (Morris, 2016). Some second-trimester abortions are induced because o etal abnormalities or patient choice.
Second-trimester losses are subclassied similarly to rst-trimester ones (p. 200). Management is similar in many regards to that used or second-trimester induced abortion, described on page 214. One exception is cervical cerclage, which may be employed or cervical insufciency.
■ Cervical Insufficiency
Previously called incompetent cervix, this is characterized classically by painless cervical dilation in the second trimester. It can be ollowed by prolapse and ballooning o the amnionic membranes into the vagina, and ultimately, expulsion o an immature etus. Tis sequence oten repeats in uture pregnancies. O causes, prior cervical trauma is implicated. One cohort study o more than 15,000 women with prior cervical conization ound a ourold risk o pregnancy loss beore 24 weeks’ gestation (Albrechtsen, 2008). However, in women solely with prior conization but no prior preterm birth, outcomes are not improved by prophylactic cerclage (Zeisler, 1997). In other instances, abnormal cervical development, including that rom diethylstilbestrol (DES), may play a role (Hoover, 2011). Mar- an and Ehlers-Danlos syndromes also carry increased risk o cervical insufciency (Meijboom, 2006; Spiegel, 2020).
Surgical Indications
For women with an unequivocal history o second-trimester painless delivery, prophylactic cerclage placement is an option and reinorces a weak cervix by an encircling suture. However,
TABLE 11-4. Clinical and Laboratory Criteria for Diagnosis of Antiphospholipid Antibody Syndromea
some women have a history and clinical ndings that make it difcult to veriy—classic cervical insufciency. In one randomized trial o almost 1300 women, cerclage was ound to be benecial—13 versus 17 percent—to prolong pregnancy past 33 weeks (MacNaughton, 1993).
Te physical nding o early dilation o the internal cervical os and visible membranes is another indicator o insufciency. In one systematic review, cerclages that were placed based on such examination ndings provided superior perinatal outcomes compared with expectant management (Ehsanipoor, 2015). Last, some use VS to determine cerclage need. In those with any prior spontaneous preterm birth, the Society or MaternalFetal Medicine (2016) recommends transvaginal cervical length screening. Te American College o Obstetricians and Gynecologists (2021) also recommends this screening. Surveillance extends between 16 and 24 weeks’ gestation, and examinations, described in Chapter 14 (p. 254), are perormed every 1 to 4 weeks. In those without prior preterm birth or in those with multietal gestation, cervical length is not specically measured.
Te cervix should be viewed during the sonographic anatomical anatomy examination at 18 to 22 weeks’ gestation. Chapter 45 (p. 794) discusses recommendations or cerclage placement based on cervical length to prevent preterm birth.
■ Presurgical Preparation
Contraindications to cerclage include bleeding, contractions, or ruptured membranes, any o which substantially raise the likelihood o labor and ailure. Prophylactic elective cerclage beore dilation is preerable, and timing between 12 and 14 weeks’ gestation allows early intervention. Still, it avoids surgery in the rst trimester, which is when most predestined spontaneous losses occur, and screening or aneuploidy and malormation is completed. Cervical neoplasia screening in suitable candidates and gonorrhea and chlamydial inection testing are done.
Obvious cervical inection is treated. At times, the cervix instead is ound to be dilated, eaced, or both, and an emergency cerclage is perormed. Notably, in more-advanced pregnancy, the risk o stimulating preterm labor or o rupturing membranes with the surgery is greater. At Parkland Hospital, cerclage procedures are not done ater 23 to 24 weeks’ gestation. Others, however, describe placement later than this (Caruso, 2000; erkildsen, 2003).
When outcomes o cerclage are evaluated, women with similar clinical presentations are ideally compared. In a study o elective cerclage by Owen and associates (2009), approximately a third o women delivered beore 35 weeks, and complications were ew. By contrast, in a 10-year review o 75 women undergoing emergency cerclage, only hal were delivered ater 36 weeks (Chasen, 1998). Importantly, only 44 percent o those with bulging membranes at the time o cerclage reached 28 weeks. erkildsen and associates (2003) had similar experiences. Our experiences at Parkland Hospital are that emergency cerclage has a high ailure rate, and women are counseled accordingly.
I the clinical indication or cerclage is questionable, a woman may instead be observed. Most undergo cervical examinations weekly or every 2 weeks to assess eacement and dilation. Unortunately, rapid eacement and dilation can develop despite such precautions (Witter, 1984).
Vaginal Cerclage
O the two vaginal cerclage operations, most use the simpler procedure developed by McDonald (1963) (Fig. 11-3). Te more complicated operation is a modication o the procedure
FIGURE 11-3 McDonald cerclage procedure for incompetent cervix. A. Start of the cerclage procedure with a no. 2 monofilament suture being placed in the body of the cervix very near the level of the internal os. B. Continuation of suture placement in the body of the cervix so as to encircle the os. C. Encirclement completed. D. The suture is tightened around the cervical canal sufficiently to reduce the diameter of the canal to 5 to 10 mm, and then the suture is tied. The effect of the suture placement on the cervical canal is apparent. A second suture placed somewhat higher may be of value if the first is not in close proximity to the internal os.
described by Shirodkar (1955)
(Fig. 11-4). When either technique is perormed electively, women with a classic history o cervical insufciency have good outcomes (Caspi, 1990; Kuhn, 1977). For either vaginal or abdominal cerclage, evidence is insufcient to recommend perioperative antibiotic prophylaxis (American College o Obstetricians and Gyne cologist, 2020a). Few data are ava ilable and do not support prophylactic tocolysis (Smith, 2015). Regional analgesia is suitable and preerred. Ater this, the woman is placed in standard lithotomy position. Te vagina and perineum are cleaned or surgery, and the bladder is drained.
Some operators do not use potentially irritating antiseptic solution i amnionic membranes are exposed and instead use warm saline (Pelosi, 1990). Although steps are described here, a thorough and illustrated review o technique is provided by Hawkins (2017).
For suturing, options include a no. 1 or 2 nylon or polypropylene monolament suture or 5-mm Mersilene tape. During placement, the suture is placed as cephalad along the cervical length as possible, is anchored into the dense cervical stroma, yet avoids the bladder. wo tandem cerclage suture rings are not more eective than one (Giraldo-Isaza, 2013).
Emergency cerclage placement with a thinned dilated cervix is more difcult, and tissue tearing and membrane puncture are risks. Gentle replacement o the prolapsed amnionic sac back into the uterus can aid suturing. Options include steep rendelenburg or lling the bladder with 600 mL o saline through a Foley catheter in the bladder. However, these steps may carry the cervix cephalad and away rom the operating eld.
Instead, membranes can be pushed inward by a wide, moist sponge stick. A Foley catheter can instead be inserted through the cervix, and ination o the 30-mL balloon can deect the amnionic sac inward. Te balloon is gradually deated as the cerclage suture is tightened around the catheter tubing, which is then removed. With any o these, simultaneous gentle outward traction created by ring orceps placed on the cervical edges may be helpul.
For uncomplicated pregnancies without labor, the cerclage is usually snipped and removed at 37 weeks’ gestation. Tis balances the risk o preterm birth against that o cervical laceration rom a cerclage in place with labor contractions. ransvaginally placed cerclages are typically removed even with cesarean delivery to avoid rare long-term oreign-body complications (Hawkins, 2014). With scheduled cesarean delivery, the cerclage may be removed at 37 weeks or deerred until the time o regional analgesia and delivery. Again, the risk o labor ensuing beore delivery must be considered. During extraction, particularly with a Shirodkar cerclage or a cerclage using Mersilene tape, analgesia aids patient comort and adequate visualization.
Transabdominal Cerclage
At times, suture at the uterine isthmus is placed abdominally. Perormed less oten than transvaginal methods, selected indications include prior transvaginal cerclage ailure or severe cervical anatomical deects. Te cerclage is let until childbearing completion, and thus cesarean delivery is required. With the cerclage in place, conception rates still approximate 75 percent (Moawad, 2018). I needed or a etal loss, dilation and evacuation can be perormed with the suture in place (Dethier, 2020).
FIGURE 11-4 Modified Shirodkar cerclage for incompetent cervix. A. A transverse incision is made in the mucosa overlying the anterior cervix, and the bladder is pushed cephalad. B. A 5-mm Mersilene tape on a swaged-on or Mayo needle is passed anterior to posterior. C. The tape is then directed posterior to anterior on the other side of the cervix. Allis clamps are placed so as to bunch the cervical tissue. This diminishes the distance that the needle must travel submucosally and aids tape placement. D. The tape is snugly tied anteriorly, after ensuring that all slack has been taken up. The cervical mucosa is then closed with continuous stitches of chromic suture.
With the procedure, ater abdomen entry, sharp dissection in the vesicocervical space allows the bladder to be pushed caudally. At the level o the internal os, a window is made in ree space medial to the uterine vessels. Tis avoids vessel compression by the tightened cerclage. Te nearby ureter is identied and avoided. One end o the ligating suture is passed into the right window, and the other is threaded into the let. Per surgeon preerence, the knot is tied either in the ront or back. Te vesicouterine peritoneum is closed with absorbable suture in a running ashion.
In the MAVRIC trial, 111 women with a prior ailed vaginal cerclage were randomly assigned to transabdominal, McDonald, or Shirodkar methods. Almost hal o the transabdominal ones were placed prior to conception. Te preterm birth rate beore 32 weeks was 8 percent in the transabdominal group and 38 percent in each o the transvaginal cerclage groups (Shennan, 2020).
O morbidity, rates o bleeding, adjacent organ injury, uterine peroration, and inection can be greater with transabdominal compared with transvaginal methods. ransabdominal cerclage perorms suitably whether laparotomy or laparoscopy is used to place the sutures (Moawad, 2018).
Complications
With cerclage in general, PPROM, preterm labor, hemorrhage, or inection is a potential risk. All are uncommon with prophylactic cerclage. In the trial by MacNaughton and associates (1993), membrane rupture complicated only 1 o more than 600 procedures done beore 19 weeks. In our view, clinical inection mandates immediate removal o the suture with labor induced or augmented. Similarly, with imminent abortion or delivery, the suture should be removed to avoid cervical laceration.
Following cerclage, sonographic surveillance does not improve outcomes (Dijkstra, 2000). Evidence does not support a subsequent reinorcing cerclage procedure (Baxter, 2005). Membrane rupture during suture placement or within the rst 48 hours ater surgery is considered by some to be an indication or cerclage removal to avoid serious etal or maternal inection (Kuhn, 1977). In those with later PPROM but without inection or labor, options include observation alone or cerclage removal and observation. In these cases, data are contradictory regarding prolonged gestational latency and inection rates with retention or removal (Jenkins, 2000; Pergialiotis, 2015). In the absence o inection or labor, we allow cerclage retention with close clinical surveillance.
INDUCED ABORTION
■ Definitions
Te term induced abortion is dened as termination o pregnancy with medication or surgery beore etal viability. Te abortion ratio is the number o abortions per 1000 live births, and abortion rate is the number per 1000 women aged 15 to 44 years. For 2018, approximately 620,000 elective abortions were reported (Kortsmit, 2020). O these, 78 percent were pregnancies aged ≤9 weeks’ gestation, and 92 percent o abortions were completed beore ≤13 weeks. Te abortion ratio was 189 per 1000 live births, and the abortion rate was 11.3. Terapeutic abortion reers to pregnancy termination or medical reasons, and suitable maternal or etal indications are described in respective chapters. In cases o rape or incest, many consider termination. Te most requent therapeutic indication currently is to prevent birth o a etus with a signicant anatomical, metabolic, or mental deormity. Te term elective abortion or voluntary abortion describes the interruption o pregnancy beore viability at the request o the woman, but not or medical reasons. Most abortions done today are elective.
■ Legal Influence
Te legality o elective abortion was established by the United States Supreme Court in the case o Roe v. Wade. Te Court dened the extent to which states might regulate abortion and ruled that rst-trimester procedures must be let to the medical judgment o the physician. Ater this, the state could regulate abortion procedures in ways reasonably related to maternal health. Last, subsequent to viability, the state could promote its interest in the potential o human lie and regulate abortion, except or preservation o the mother’s health.
Other legislation ollowed. Te 1976 Hyde Amendment orbids use o ederal unds to provide abortion services except in cases o rape, incest, or lie-threatening circumstances. Te Supreme Court in 1992 reviewed Planned Parenthood v. Casey and upheld the undamental right to abortion, but established that regulations are constitutional as long as they do not impose an “undue burden” on the woman. Subsequently, many states introduced counseling requirements, waiting periods, parental consent or minors, acility requirements, and unding restrictions. Such limits are oten called targeted regulation o abortion providers (RAP) laws. One major choice-limiting decision was the 2007 Supreme Court decision that reviewed Gonzales v. Carhart and upheld the 2003 Partial-Birth Abortion Ban Act. In 2016, some RAP laws were dialed back by the Supreme Court ruling in the case o Whole Woman’s Health v. Hellerstedt. With this, the justices noted that abortion laws must coner health saety benets that outweigh burdens on access.
■ Provider Availability
Major women’s health organizations support the legal right o women to obtain an abortion (Espey, 2019). Te Accreditation Council or Graduate Medical Education mandates that obstetrics and gynecology residency education must include access to experience with induced abortion. Te Kenneth J. Ryan Residency raining Program was established in 1999 to work with residency programs to improve abortion and contraceptive training. Moreover, postresidency training in these techniques is available in ormal 2-year Family Planning ellowships. Other residency programs have a less-codied abortion curriculum. Instead, residents learn technical aspects through their management o miscarriage and pregnancy interruption or medical indications.
Te American College o Obstetricians and Gynecologists (2019g) respects the need and responsibility o health-care providers to determine their individual positions on induced abortion. It also advocates or counseling and timely reerral i providers have individual belies that preclude pregnancy termination. Knowledgeable and compassionate counseling objectively describes and provides inormation to the woman to permit inormed decision-making.
FIRST-TRIMESTER METHODS
Abortions can be completed with either medication or surgery. In the absence o serious maternal disorders, abortion procedures do not require hospitalization (Guiahi, 2012). However, outpatient acilities should be able to provide emergency resuscitation and immediate transer to a hospital (Levy, 2019).
■ Surgical Abortion
Preoperative Preparation
Surgical evacuation is perormed transvaginally through an appropriately dilated cervix. For this, preoperative cervical ripening is typically associated with easier intraoperative cervical dilation, less pain, a technically easier procedure, and shorter operative times (Kapp, 2010; Webber, 2015). On balance, this preparation adds a surgical delay and potential side eects. Tus, cervical priming in the rst trimester may be reserved or those with anticipated challenges to dilation. Examples are those with cervical stenosis or adolescents, who overall may experience higher associated pain (Allen, 2016). Surgical steps presented here apply to both induced abortion and miscarriage. For ripening, hygroscopic dilators, also called osmotic dilators, are devices that draw water rom surrounding tissues and expand gradually to dilate the endocervical canal. One type is derived rom various species o Laminaria algae that are harvested rom the ocean oor (Fig. 11-5). Tese come in dierent diameters, which allow the number and diameters o inserted devices, also called tents, to be customized to a given cervix. Another device is Dilapan-S, which is composed o an acrylicbased gel. Each type expands to an ultimate diameter three to our times that o its dry state. With hygroscopic dilators, shallow insertion yields insufcient dilation or tent expulsion. Overly deep placement risks dislodgement into the uterine cavity. Once tents are inserted, several gauze sponges placed at the external os help prevent spontaneous tent expulsion. Patients can ambulate, void, or stool without limitation. Te numbers o sponges and dilators inserted are careully counted and recorded in the chart.
Schneider and coworkers (1991) described 21 cases in which women who had a hygroscopic dilator placed changed their minds. O 17 women who chose to continue their pregnancy, 14 delivered at term, two delivered preterm, and one miscarried 2 weeks later. None suered inection-related morbidity, including three untreated women with cervical cultures positive or Chlamydia trachomatis.
Instead, misoprostol is oten used or cervical ripening. Te typical dose is 400 μg administered sublingually, buccally, or placed into the posterior vaginal ornix at least 3 to 4 hours prior to surgery. Oral ingestion is less eective (Allen, 2016). Another option is a 200-mg oral miepristone dose given 24 to 48 hours beore surgery (Ashok, 2000). However, misoprostol is typically avored, because o miepristone’s greater delay to the procedure, cost, and limited access within the FDA’s REMS program (p. 202).
O options, hygroscopic dilators provide equal or slightly greater dilation than misoprostol. Other surgical parameters do not vary signicantly (Bartz, 2013; MacIsaac, 1999). On balance, the ripening time required or hygroscopic dilators extends the total procedure time and can be uncomortable, whereas misoprostol can cause ever, bleeding, and gastrointestinal side eects. I not done as part o early prenatal care, hemoglobin level and Rh status are assessed prior to abortion. Screening or gonorrhea, or syphilis, and or human immunodeciency virus, hepatitis B, and chlamydial inections also is completed. Obvious cervical inections are treated and resolved beore elective procedures. o prevent postabortal inection ater a rst- or secondtrimester surgical evacuation, a 200-mg oral prophylactic dose
FIGURE 11-5 Hygroscopic dilators. With each type, the dry unit (left) expands exponentially when exposed to water (right) as in the endocervical canal. A. Laminaria. B. Dilapan-S.
o doxycycline is given 1 hour beore. For those electing local anesthesia, evidence also supports adding an oral or intramuscular dose o a nonsteroidal antiinammatory drug 30 to 60 minutes prior to surgery (Allen, 2018). Prophylaxis speci- cally or inective endocarditis prevention in those with valvular heart disease is not required in the absence o active inection (American College o Obstetricians and Gynecologists, 2020g,i). No recommendations specically address venous thromboembolism prophylaxis or suction curettage in low-risk gravidas. At our hospital, we encourage early ambulation.
Vacuum Aspiration
Also called suction curettage or suction dilation and curettage, vacuum aspiration is a transcervical approach, in which the cervix is rst dilated and tissue is then evacuated. For this, a rigid cannula is attached either to an electric-powered vacuum source or to a handheld 60-mL syringe or its vacuum source. Tese are electric vacuum aspiration (EVA) or manual vacuum aspiration (MVA), respectively. Sharp dilation and curettage (D & C) in which contents are mechanically scraped out solely by a sharp curette is currently not recommended or pregnancy evacuation due to greater blood loss, pain, and procedural time (National Abortion Federation, 2020; World Health Organization, 2012). Importantly, this practice is distinguished rom brie nal sharp curettage ollowing initial aspiration. o begin, the surgeon perorms a bimanual examination to conrm uterine size and orientation. A speculum is inserted, and the cervix is swabbed with povidone-iodine or equivalent solution. Te anterior cervical lip is grasped with a toothed tenaculum. Te cervix, vagina, and uterus are richly supplied by nerves o the Frankenhäuser plexus, which lies within connective tissue lateral to the uterosacral and cardinal ligaments.
Tus, vacuum aspiration at minimum requires intravenously or orally administered sedatives or analgesics, and some add a paracervical or intracervical block (Allen, 2009; Renner, 2012). For a pudendal block, 5 mL o 1- or 2-percent lidocaine is injected into the uterosacral ligaments at their insertion into the uterus at 4 and 8 o’clock. Instead, an intracervical block with 5-mL aliquots o 1-percent lidocaine injected at 12, 3, 6, and 9 o’clock was reported to be equally eective (Mankowski, 2009). General or regional anesthesia may instead be elected.
First, a Sims uterine sound is passed into the uterus to measure the depth and inclination o the cavity. Tis provides parameters or subsequent instrument insertion. I required, the cervix is urther dilated with Hegar, Hank, or Pratt dilators until a suction cannula can be inserted. As a rough rule, the degree o required cervical dilation in millimeters approximates gestational age. Hegar sizes reect their diameter in millimeters. Pratt and Hank dilators are sized in French units, which can be converted to millimeters by dividing the French value by three. With dilation, the ourth and th ngers o the introducing hand should rest on the perineum and buttocks as the instrument is guided through the internal os (Fig. 11-6). Tis technique minimizes orceul insertion and helps prevent uterine peroration.
Following dilation, or most rst-trimester aspiration procedures, an 8- to 12-mm Karman cannula is appropriate. Small cannulas carry the risks o a longer surgery and o missed intrauterine tissue. Large cannulas risk cervical injury and more discomort. For evacuation, the cannula is slowly moved toward the undus until resistance is met. Suction is then activated. Te cannula is gradually pulled back toward the os and is simultaneously slowly turned circumerentially to cover the entire uterine cavity surace (Fig. 11-7). Tis is repeated until no more tissue is aspirated. A gentle sharp curettage can ollow to remove any remaining tissue ragments (Fig. 11-8). Strong and consistent evidence supports the high efcacy, saety, and patient acceptability or both MVA and EVA (Lichtenberg, 2013).
For abortion done at ≤6 weeks’ gestation, a distinct drawback is that the pregnancy may be small and missed by the curette. o identiy placenta, the aspirated contents are rinsed in a strainer to remove blood, and then placed in a clear plastic container with saline and examined with back lighting (MacIsaac, 2000). Placental tissue macroscopically appears eathery. A magniying lens, colposcope, or microscope can augment viewing. With gestations <6 weeks, the ailed abortion rate approximates 2 percent (Paul, 2002). Tus, i products are not clearly identied, serial serum β-hCG levels can be inormative (Dean, 2015).
Abortion Complications
Legally induced abortion in the United States has a low associated mortality rate, and rom 2013 to 2017, the rate was 0.4 deaths in 100,000 procedures (Kortsmit, 2020). Early abortions are saer. Te mortality rate was 0.3 deaths in 100,000 procedures perormed ≤8 weeks’ gestation but rose to 2.5 at 14 to 17 weeks and to 6.7 at ≥18 weeks (Zane, 2015). Notably, maternal mortality rates are 14-old greater or pregnancies that are continued (Raymond, 2012). Uterine peroration and lower-genital-tract laceration are uncommon but potentially serious, and rates also rise with
FIGURE 11-6 Dilation of cervix with a Hegar dilator. Note that the fourth and fifth fingers rest against the perineum and buttocks, lateral to the vagina. This maneuver is an important safety measure because if the cervix relaxes abruptly, these fingers prevent a sudden and uncontrolled thrust of the dilator, a common cause of uterine perforation.
gestational age. In one systematic review o rst-trimester abortion, the uterine peroration and laceration rates were each ≤1 percent (White, 2015). Peroration is usually recognized when the instrument passes without resistance deep into the pelvis. Risk actors include operator inexperience, prior cervical surgery or anomaly, adolescence, multiparity, and advanced gestational age (Allen, 2016; Grimes, 1984). I the uterine per- oration is small and undal, as when produced by a uterine sound or narrow dilator, observation or vital sign changes or uterine bleeding is usually sufcient.
I a suction cannula or sharp curette passes into the peritoneal cavity, considerable intraabdominal damage can ensue. Laparotomy or laparoscopy to thoroughly examine the abdominal contents is oten the saest course. Ater potential injuries are resolved, intraoperatively, curettage can be completed under the direct guidance provided by laparoscopy or laparotomy (Owen, 2017).
Following curettage, uterine synechiae may orm, and the risk o synechiae increases with procedure number. Most cases are mild and o unclear reproductive signicance (Hooker, 2014). However, o Asherman syndrome cases, one series ound that two thirds were linked to rst-trimester curettage (Schenker, 1982).
Other rst-trimester abortion complications are germane to both surgical and medication abortion techniques. First, hemorrhage with abortion is variably dened. One supported by the Society or Family Planning is bleeding that prompts a clinical response or bleeding in excess o 500 mL (Kerns, 2013).
For rst-trimester surgical abortions, hemorrhage complicates ≤1 percent (White, 2015). Atony, abnormal placentation, and coagulopathy are requent sources, whereas surgical trauma is a rare cause. With medication abortion, bleeding is more common. In one study o more than 42,000 Finnish women undergoing pregnancy termination with pregnancies less 63 days, hemorrhage complicated 15 percent o medication abortion but only 2 percent o surgical cases (Niinimäki, 2009).
Inection is another risk. One review ound a cumulative rate o 0.5 percent in those given prophylaxis compared with 2.6 percent in those given placebo (Achilles, 2011). In another review, the postoperative inection rate was <0.3 percent or either surgical or medication abortion (Upadhyay, 2015).
Incomplete abortion may require reevacuation. For medication abortion, this neared 5 percent in one systematic review (Raymond, 2013). Reaspiration rates ollowing surgical abortion are typically <2 percent (Ireland, 2015; Niinimaki, 2009). In sum, surgical abortion oers higher efcacy rates (96 to 100 percent) than medication abortion (83 to 98 percent) in the rst trimester. Medication abortion also carries a greater cumulative risk o complications, although dierences are small (Lichtenberg, 2013). Tese are balanced against the greater privacy o medication abortion and the more invasive steps o curettage.
■ Medication Abortion
Agents Used
For many women, outpatient medication abortion is an acceptable option or pregnancies with a menstrual age ≤70 days. Although suitable at later gestational ages, successul evacuation rates are lower. Among abortions perormed at ≤9 weeks’ gestation, 39 percent were completed with medication in the United States
FIGURE 11-7 A suction curette has been placed through the cervix into the uterus. The figure shows the rotary motion used to aspirate the contents. (Figures 11-7 and 11-8: Reproduced with permission from Hoffman BL, Hamid CA, Corton MM: Surgeries for benign gynecologic disorders. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, McGraw Hill Education, 2020.)
FIGURE 11-8 A sharp curette is advanced into the uterine cavity while the instrument is held with the thumb and forefinger as shown in Figure 11-6. Upon reaching the fundus, the curette is placed flush against the uterine wall. Firm pressure against the wall as the instrument is dragged out will scrape away adhered tissue fragments. With movement of the curette, only the strength of these two fingers should be used to help avoid perforation.
in 2018 (Kortsmit, 2020). One regimen provides miepristone plus misoprostol, but another provides misoprostol alone. O actions, miepristone augments uterine contractility by reversing progesterone-induced myometrial quiescence. Misoprostol directly stimulates the myometrium. Both also ripen the cervix (Mahajan, 1997; ang, 2007).
Contraindications have evolved rom exclusion criteria that were used in clinical trials. Cautions include suspected ectopic pregnancy; in-situ intrauterine device; severe anemia, coagulopathy, or anticoagulant use; long-term systemic corticosteroid therapy; chronic adrenal ailure; inherited porphyria; or allergy to agents used (American College o Obstetricians and Gynecologists, 2020c). O note, misoprostol is suitable or early pregnancy ailure in those with prior uterine surgery (Chen, 2008).
Misoprostol is a teratogen that is associated with transverse limb reduction or with the Mobius sequence, which is maniest by cranial nerve palsy. Tus, a commitment to abortion completion is essential once this drug is given (Vauzelle, 2013).
With miepristone, or women who choose to continue their pregnancies ater exposure, the ongoing pregnancy rate ranges rom 10 to 46 percent (Grossman, 2015). Te associated major malormation rate was 5 percent in one series o 46 exposed pregnancies (Bernard, 2013).
Administration
Several dosing schemes are eective (Table 11-6). Because o its greater efcacy, miepristone plus misoprostol combinations are avored. For gestations ≤70 days, the most widely accepted regimen is miepristone, 200 mg given orally on day 0. Tis is ollowed in 24 to 48 hours by an 800-μg misoprostol dose that is administered vaginally, buccally, or sublingually (American College o Obstetricians and Gynecologists, 2020c).
Oral ingestion has lower efcacy rates. I desired, miepristone and misoprostol may be sel-administered at home (Gambir, 2020). Associated inection rates approximate 0.3 percent, and antibiotic prophylaxis is not currently recommended (Achilles, 2011).
Symptoms ollowing misoprostol are common within 3 hours and include vomiting, diarrhea, ever, and chills. Bleeding and cramping with medication abortion typically is signi- cantly worse than that with menses. Tus, oral analgesics are provided. I bleeding soaks two or more pads in an hour or at least 2 hours, the woman is instructed to contact her provider.
Reappointment is 1 to 2 weeks ollowing drug administration, and bimanual pelvic examination is recommended. Abortion completion may be assessed with β-hCG levels. Expected rates o decline at 1 week can be extrapolated rom able 11-1 and can guide care. However, specic β-hCG values lack suitable sensitivity (Rørbye, 2004). Moreover, routine postabortal sonographic examination is typically unnecessary (Clark, 2010). I done, it perorms comparably to β-hCG level assessment (Dayananda, 2013). I sonography is done due to concern or ailed abortion or or bleeding, unnecessary surgery can be avoided i scans are interpreted appropriately. I no gestational sac is seen and no heavy bleeding is noted, intervention is unnecessary. Tis is true even when, as is common, the uterus contains sonographically evident debris (Reeves, 2008).
TABLE 11-6. Various Regimens for Medical Termination of Pregnancy
then escalate sequentially in a similar fashion through 150, 200, 250, and finally 300 units oxytocin, each in 500 mL normal saline
aWith misoprostol, efficacy is similar for vaginal, buccal, and sublingual routes, whereas oral ingestion is less effective.
bIf abortion not completed by fifth dose, the cycle may be repeated following a 24-hour rest period.
American College of Obstetricians and Gynecologists, 2019f, 2020c; Raymond, 2019; Schreiber, 2018; Whitehouse, 2020; World Health Organization, 2018.
SECOND-TRIMESTER METHODS
In the second trimester, etal anomaly or death, maternal health complications, inevitable abortion, or desired termination may be indications or uterine evacuation. As in the rst trimester, medication or surgery is an option. In the second trimester, dilation and evacuation (D & E) rather than suction D & C is needed because o larger etal size and bones.
O options, D & E is a common means in the United States. O all induced abortions in 2018, nearly 8 percent were per- ormed by D & E at gestational ages >13 weeks (Kortsmit, 2020). Many o the surgical and medication steps or secondtrimester abortion mirror those in the rst trimester, and di- erences are emphasized here.
■ Dilation and Evacuation
Preparation
With D & E, wide mechanical cervical dilation is needed or evacuation o etal parts. Te degree needed rises with etal gestational age, and inadequate dilation risks cervical trauma, uterine peroration, or tissue retention (Peterson, 1983). Tus, presurgical cervical preparation is advised, and main options include hygroscopic dilators or misoprostol (p. 209). With laminaria, overnight preparation oers optimal cervical dilation (Fox, 2014). Uncommonly, laminaria may ail to adequately dilate the cervix, and serial insertions over several days with an increasing number o tents is an option. Dilapan-S also is suitable. It may be preerable or same-day procedures, as this device achieves its maximal eect in 4 to 6 hours (Newmann, 2014). Supplementing laminaria with miepristone can aid procedures or gestations >19 weeks (Diedrich, 2020; Goldberg, 2015). In contrast, misoprostol supplementation to hygroscopic dilators ailed to add benets in one metaanalysis (Cahill, 2020).
I a patient changes abortion plans ater hygroscopic dilator removal, preterm delivery and PPROM rates are substantial. In one series o 12 such cases, 50 percent ended with miscarriage or perinatal death (Mark, 2019).
Misoprostol alone can be used or cervical preparation. Te typical dose is 400 μg given vaginally or buccally 3 to 4 hours prior to D & E. Randomized trial results vary regarding the ability o misoprostol to achieve results equal to that with hydroscopic dilators (Bartz, 2013; Goldberg, 2005; Sagiv, 2015). In women with one prior hysterotomy, the risk o uterine rupture was not elevated by misoprostol cervical ripening in one review but rose to 2.5 percent in those with ≥2 cesarean deliveries (Andrikopoulou, 2016).
With miepristone alone or cervical ripening, ewer studies provide data. In one, miepristone alone provided less dilation than hydroscopic dilators (Borgatta, 2012). In another trial, miepristone given 48 hours beore misoprostol created greater cervical dilation compared with misoprostol alone (Carbonell, 2007).
In sum, hygroscopic dilators soten and dilate the cervix beore D & E. Sequential insertions or layering agents may be most helpul or later gestations or or an inadequate response to initial hygroscopic dilators alone. Yet, layering adds cost and potential side eects.
With elective abortion, some choose to induce etal demise prior to D & E to avert a live birth. For this, an intracardiac potassium chloride or lidocaine injection or an intraamnionic or intraetal digoxin injection is used prior to cervical ripening (ua, 2020).
Technique
During D & E, sonography can be used as an adjunct in all cases or selectively in more challenging ones. Perioperative antibiotic prophylaxis mirrors that or rst-trimester procedures (p. 209). o reduce postprocedure bleeding, dilute vasopressin can be injected intracervically or as part o a paracervical block (Kerns, 2013; Schulz, 1985). Once adequate cervical dilation is achieved, the initial surgical step drains amnionic uid with an 11- to 16-mm suction cannula or with amniotomy. Tis reduces the risk o amnionic uid embolism and brings the etus into the lower uterine segment or removal (Owen, 2017; Prager, 2009).
For pregnancies >16 weeks’ gestation, the etus is extracted, oten in parts, using Sopher orceps or other destructive instruments. With complete removal o the etus, a large-bore vacuum curette is used to remove the placenta. Major complications are inrequent with D & E, and rates range rom 1 to 2 percent in large series (Lederle, 2015; Peterson, 1983). Tese include uterine peroration, cervical laceration, uterine bleeding, and postabortal inection. Prior cesarean delivery is not a contraindication or D & E and may be pre- erred over prostaglandins or those with multiple prior hysterotomies (Ben-Ami, 2009).
Other Surgical Considerations
Placenta previa or the placenta accreta syndrome (PAS) can raise D & E risks. Once diagnosed, PAS typically prompts hysterectomy (Matsuzaki, 2015). For placenta previa, D & E is preerred to quickly evacuate the placenta, but the ability to transuse blood products and perorm possible hysterectomy must be available (American College o Obstetricians and Gynecologists, 2019; Perriera, 2017). Medication abortion may be elected, but the risk or transusion is greater than with D & E (Nakayama, 2007). Data are conicting regarding the value o predelivery uterine artery embolization to lessen bleeding risks (Wang, 2019).
In some cases o ailed second-trimester medication abortion, hysterotomy may be considered. In other cases, i comorbid uterine pathology is signicant, such as numerous large myomas, hysterectomy may provide ideal treatment. Some women with second-trimester pregnancies desire sterilization ollowing evacuation. Because the contracted uterine undus will lie lower, any laparotomy incision must be placed to allow allopian tube access.
■ Medication Abortion
Principal among noninvasive methods is a miepristone plus misoprostol regimen or misoprostol alone (see able 11-6). O these two options, the combined regimen yields a shorter termination duration (Kapp, 2007; Ngoc, 2011). Hygroscopic dilators may speed the time to delivery with this combined regimen
(Mazouni, 2009; Vincienne, 2018). In selecting misoprostol routes, oral administration leads to a longer time to delivery compared with vaginal or sublingual routes (Dickinson, 2014). Prophylactic antibiotics are not typically given, and inection surveillance during labor is instead applied (Achilles, 2011). Another induction agent, PGE2, shows similar efcacy and side eects compared with misoprostol (Jain, 1994; Jansen, 2008). Simultaneous administration o an antiemetic such as metoclopramide (Reglan), an antipyretic such as acetaminophen, and an antidiarrheal such as diphenoxylate/atropine (Lomotil) will help prevent or treat symptoms. Dinoprostone (Prostin) is an available PGE2 in the United States. However, its greater cost and poor pharmacologic stability at room temperature may make it less attractive than misoprostol.
During medication abortion, the uterine rupture rate is 0.4 percent with misoprostol and one prior low transverse cesarean delivery (Berghella, 2009). Both misoprostol and PGE2 appear to pose similar risk (le Roux, 2001; Reichman, 2007). Few data guide their use or medication abortion in those with ≥2 prior cesarean deliveries.
O other agents, high-dose intravenous oxytocin in saline will result in second-trimester abortion in 80 to 90 percent o cases (see able 11-6). However, by comparison, misoprostol leads to higher successul induction rates and aster delivery times (Alavi, 2013).
Rarely used in the United States, ethacridine lactate is an organic antiseptic that activates myometrial mast cells to release prostaglandins (Olund, 1980). Te solution is instilled either intraamnionically or extraovularly, that is, into the potential space between the uterine wall and amnion. Compared with misoprostol, it is associated with longer times to delivery and lower success rates (Hou, 2011).
■ Fetal and Placental Evaluation
For second-trimester pregnancies being terminated or maternal health or etal indications, either D & E or medication abortion is suitable. Tus, patient input and clinical indication both guide selection. Perinatal palliative care can be instituted (p. 203) (Marc-Aurele, 2020). Evaluation o a stillborn etus is described in Chapter 35 (p. 626). One component is autopsy, which can also be valuable or second-trimester terminations due to anomalies (Hauerberg, 2012; Man, 2016). Fragmented D & E specimens may provide less inormation than intact etuses (Lal, 2014).
ELECTIVE ABORTION CONSEQUENCES
Data relating abortion to overall maternal health and to subsequent pregnancy outcome are limited. Rates o inertility or ectopic pregnancy are not increased (Burkman, 1988; Frank, 1993). Exceptions may stem rom postabortal inections, especially those caused by C trachomatis. O subsequent adverse pregnancy outcomes, several studies note an approximate 1.5- old greater incidence o preterm delivery ollowing surgical evacuation (Lemmers, 2016; Saccone, 2016). Tis risk accrues with the number o terminations (Klemetti, 2012). Subsequent pregnancy outcomes are similar i a prior induced abortion was completed with medication or by surgery (Männistö, 2013; Virk, 2007).
POSTABORTAL CONTRACEPTION
Ater early abortion, woman may ovulate ater 8 days, but the average is 3 weeks (Lahteenmaki, 1978; Stoddard, 2011). Unless another pregnancy is imminently desired, contraception, either hormonal or nonhormonal, can be started immediately ater abortion (Curtis, 2016). In candidates, dened in Chapter 38 (p. 667), an intrauterine device can be inserted immediately ater surgery or medication abortion is completed (Bednarek, 2011; Korjamo, 2017).
For women who desire another pregnancy, conception need not be delayed. From studies, groups conceiving within 3 months o a rst-trimester loss had lower subsequent miscarriage rates compared with groups with later conceptions (Schliep, 2016).

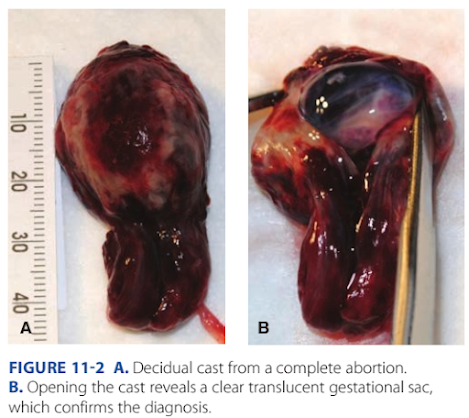











Nhận xét
Đăng nhận xét