Chapter 12. Ectopic Pregnancy
BS. Nguyễn Hồng Anh
Following ertilization and allopian tube transit, the blastocyst normally implants in the endometrial lining o the uterine cavity. Implantation elsewhere is considered ectopic. In
the United States, numbers rom an insurance database and
rom Medicaid claims showed ectopic pregnancy rates o 1.54
percent and 1.38 percent, respectively, in 2013 (ao, 2017).
Ectopic implantation accounts or 3 percent o all pregnancyrelated deaths (Creanga, 2017). Fortunately, beta-human
chorionic gonadotropin (β-hCG) assays and transvaginal
sonography (VS) aid earlier diagnosis, maternal survival, and
ertility conservation.
TUBAL PREGNANCY
■ Classfcaton
O ectopic pregnancies, nearly 95 percent implant in the allopian tube’s various segments (Fig. 2-13, p. 26). Te ampulla
(70 percent) is the most requent site (Fig. 12-1). Te rate or
isthmic implantation is 12 percent; mbrial, 11 percent; and
interstitial, 2 percent (Bouyer, 2002). Nontubal ectopic pregnancies compose the remaining 5 percent and implant in the
ovary, peritoneal cavity, cervix, or prior cesarean scar. Occasionally, a multietal pregnancy contains one conceptus with
normal uterine implantation and the other implanted ectopically. Tis is termed a heterotopic pregnancy (p. 231).
For all ectopic pregnancy sites, management is inuenced by
pregnancy viability, gestational age, maternal health, desires or
the index pregnancy and or uture ertility, physician skill, and
available resources. Regardless o location, D-negative women
with an ectopic pregnancy are given anti-D immunoglobulin.
In rst-trimester pregnancies, a single intramuscular 50- or
120-μg dose is appropriate. Later gestations are given 300 μg
(American College o Obstetricians and Gynecologists, 2019b).
■ Rsks
Abnormal allopian tube anatomy underlies most cases o tubal
ectopic pregnancy. Surgeries or a prior tubal pregnancy, or
ertility restoration, or or sterilization coner the highest risk.
Ater one prior ectopic pregnancy, the chance o another nears
10 percent (de Bennetot, 2012). Previous tubal inection, which
can distort normal tubal anatomy, is another risk. Specically,
one episode o salpingitis can be ollowed by a subsequent ectopic pregnancy in up to 9 percent o women (Westrom, 1992).
Peritubal adhesions that orm rom salpingitis, appendicitis, or
endometriosis also raise chances.
Inertility and the use o assisted reproductive technologies
(AR) to overcome it are linked to increased ectopic pregnancy
rates (Li, 2015; Perkins, 2015). Newer techniques aim to lower
this rate with AR (Londra, 2015; Zhang, 2017). Smoking
is another known association, although the underlying mechanism is unclear (Hyland, 2015). Last, with any orm o contraception, the absolute number o ectopic pregnancies declines
because pregnancy is eectively prevented. However, some
methods more efciently prevent intracavity implantation and
with their ailure, ectopic implantation is avored. Tese methods are tubal sterilization, intrauterine devices (IUDs), and
progestin-only contraceptives.
■ Pathogeness and Potental Outcomes
With tubal pregnancy, because the allopian tube lacks a submucosal layer, the ertilized ovum promptly burrows through
the epithelium. Te zygote comes to lie near or within the muscularis, which is invaded by rapidly prolierating trophoblast.
Potential outcomes rom this include tubal rupture, tubal abortion, or pregnancy ailure with resolution.
With rupture, the invading and expanding conceptus can
tear the allopian tube. ubal ectopic pregnancies usually rupture spontaneously but may occasionally burst ollowing coitus
or bimanual examination. Hemorrhage usually persists and can
become lie threatening.
Tubal abortion describes the pregnancy’s passage out the allopian tube’s distal end. Subsequently, hemorrhage may cease,
and symptoms eventually disappear. However, bleeding instead
can progress to induce symptoms as long as products remain
in the tube. Blood slowly issues rom the tubal mbria into
the peritoneal cavity and pools in the rectouterine cul-de-sac.
I the mbriated extremity is occluded, the allopian tube may
gradually distend with blood to orm a hematosalpinx. Rarely,
an aborted etus will secondarily implant on a peritoneal surace
and become an abdominal pregnancy (p. 231).
Last, spontaneous ailure reects ectopic pregnancy death and
subsequent reabsorption. Tese are now more regularly identi-
ed by current sensitive β-hCG assays and surveillance.
Distinctions between acute ectopic pregnancy, just described,
and chronic ectopic pregnancy also can be drawn. Acute ectopic
pregnancies are more common, produce a high serum β-hCG
level, and grow rapidly, leading to a timely diagnosis. Tese
carry a greater risk o rupture (Barnhart, 2003c). With chronic
ectopic pregnancy, abnormal trophoblasts die early, and thus
serum β-hCG levels are negative or are low and static. Chronic
ectopic pregnancies typically rupture late, i at all, but commonly orm a persistent complex pelvic mass. Tis sonographic
nding, rather than patient symptoms, oten is the reason that
prompts diagnostic surgery (emper, 2019).
■ Clncal Manfestatons
Sources o abdominal pain during pregnancy are extensive.
Uterine conditions include miscarriage, inection, degenerating
or enlarging leiomyomas, or round-ligament pain. Adnexal pain
may reect ectopic pregnancy or ovarian masses that are hemorrhagic, ruptured, or torsed. Appendicitis, renal stone, cystitis, and gastroenteritis are some nongynecological reasons or
lower abdominal pain in early pregnancy. Tus, an initial urine
β-hCG assay, urinalysis, and measure o hemoglobin or hematocrit are routine. A complete blood count (CBC) to assess the
white blood cell count may be preerred i serious inection is a
possible diagnosis. A positive urine pregnancy test result should
prompt a serum β-hCG assay or those with pain or bleeding.
Beore rupture, symptoms and signs o ectopic pregnancy
are oten subtle or absent. Te classic triad is amenorrhea that
is ollowed by pain and vaginal bleeding. With tubal rupture,
lower abdominal and pelvic pain is usually severe and requently described as sharp, stabbing, or tearing. Some degree
o vaginal spotting or bleeding is reported by most women with
tubal pregnancy. Although prouse vaginal bleeding suggests an
incomplete abortion, such bleeding occasionally is seen with
tubal gestations. Moreover, tubal pregnancy can lead to signi-
cant intraabdominal hemorrhage. Neck or shoulder pain, especially on inspiration, develops in women with diaphragmatic
irritation rom a sizable hemoperitoneum. Vertigo and syncope
may reect hemorrhage-related hypovolemia.
O physical ndings, abdominal palpation elicits tenderness.
Bimanual pelvic examination may reveal a mass and tenderness,
but this examination should be limited and gentle to avoid iatrogenic rupture. Te uterus itsel can be slightly enlarged due to
hormonal stimulation. Responses to moderate bleeding include
no change in vital signs, a slight rise in blood pressure, or a
vasovagal response with bradycardia and hypotension. Blood
pressure will all and pulse will rise only i bleeding continues
and hypovolemia becomes signicant.
O laboratory ndings, hemoglobin or hematocrit readings
may at rst show only a slight reduction, even ater substantive
hemorrhage. Tus, ater acute hemorrhage, a trending decline
in hemoglobin or hematocrit levels over several hours is a more
valuable index o blood loss than is the initial level. In approximately hal o women with a ruptured ectopic pregnancy, varying degrees o leukocytosis may reach 30,000/μL.
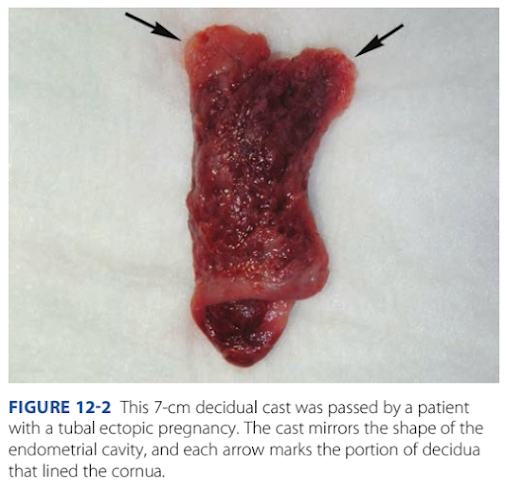
Decidua is endometrium that is hormonally prepared or
pregnancy. Te degree to which the endometrium is converted
with ectopic pregnancy varies. Tus, in addition to bleeding,222 First- and Second-Trimester Pregnancy Loss
Section 5
women with ectopic tubal pregnancy may pass a decidual cast.
Tis is the entire sloughed endometrium that takes the orm
o the endometrial cavity (Fig. 12-2). Importantly, decidual
sloughing may also occur with miscarriage. Tus, tissue is care-
ully examined by the provider and then submitted to evaluate
or histological evidence o a conceptus. I no clear gestational
sac is seen by inspection or i no villi are identied histologically,
the possibility o ectopic pregnancy must still be considered.
TUBAL PREGNANCY DiAGNOSiS
For ectopic pregnancy, physical ndings, serum β-hCG level
measurement, VS, and at times diagnostic surgery are tools
or diagnosis. Women with evidence o tubal rupture undergo
prompt surgery. For all other hemodynamically stable women
without a clearly identied pregnancy, diagnostic strategies use
these tools to identiy ectopic pregnancy.
Strategies involve trade-os. Tose that maximize ectopic
pregnancy detection may terminate a normal intrauterine pregnancy (IUP). Conversely, those that reduce the potential interruption o a normal IUP can delay ectopic pregnancy diagnosis.
Patient desires or the index pregnancy are sought and inuence
these trade-os.
■ BetaHuman Choronc Gonadotropn
Rapid and accurate determination o pregnancy is a undamental
step. hCG is a glycoprotein produced by placental trophoblast
and can be detected in serum in early pregnancy. Current pregnancy tests are immunoassays that seek the beta subunit o hCG.
Lower limits o detection are 20 to 25 mIU/mL or urine and
≤5 mIU/mL or serum (Greene, 2015). Dierent assays can have
results that vary by 5 to 10 percent. Tus, serial values are more
reliable when perormed by the same laboratory (Desai, 2014).
For women with a positive pregnancy test result plus bleeding
or pain, an initial VS is typically perormed to locate the gestation. Te initial β-hCG level sets expectations or anticipated
VS nding. With values above a discriminatory threshold,
a normal IUP is expected to be seen within the uterus. Some
institutions set their discriminatory threshold at ≥1500 mIU/
mL, whereas others use ≥2000 mIU/mL. Connolly and associates (2013) suggested an even higher threshold. Tey noted that
with live IUPs, a gestational sac was seen 99 percent o the time
in those with a discriminatory level >3510 mIU/mL.
■ Transvagnal Sonography
Pregnancy of Unknown Location
I a yolk sac, embryo, or etus is ound within the uterus or within
the adnexa, a diagnosis is made. However, i no evidence o an
IUP is seen with VS, the diagnosis is a pregnancy o unknown
location (PUL). Most PULs reect: (1) a ailing IUP, (2) recent
completed abortion, (3) early IUP, or (4) ectopic pregnancy.
Without clear evidence or ectopic pregnancy, serial β-hCG
level assessment is reasonable, and a second level is obtained 48
hours ater the rst. Tis practice averts unnecessary methotrexate therapy and avoids harming an early, normal IUP.
With early, normal IUPs, Barnhart and coworkers (2004b)
reported a 53-percent minimum rise over 48 hours. Seeber and
colleagues (2006) ound an even more conservative minimal
35-percent rise in normal IUPs. With multietal gestation, this
same anticipated rate o rise is expected (Chung, 2006).
With a PUL ultimately diagnosed as a ailed IUP, a pattern
o β-hCG level decline also can be anticipated, and levels drop
rapidly (able 11-1, p. 202) (Barnhart, 2004a). Sometimes,
PULs ail beore their location is identied. With ailing PULs,
Butts and coworkers (2013) ound rates o decline that ranged
rom 35 to 50 percent at 48 hours and 66 to 87 percent at 7
days or starting hCG values between 250 and 5000 mIU/mL.
Despite these benchmarks, a third o women with an ectopic pregnancy can also have a 53-percent rise at 48 hours (Silva,
2006). Overall, approximately hal o ectopic pregnancies show
declining β-hCG levels, whereas the other hal have rising levels. Importantly, despite a declining β-hCG level, a resolving
ectopic pregnancy may rupture. Rupture at low values likely
reects partial disruption o the vascular connection between
trophoblast and maternal vessels. Here, although β-hCG is
produced, it is unable to enter circulation and be detected.
Ater the initial two β-hCG tests during PUL assessment,
additional levels are drawn every 2 to 7 days. In general, testing
is typically more requent i symptoms or β-hCG level trends
reect a higher ectopic pregnancy risk (American College o
Obstetricians and Gynecologists, 2019c). VS also may be
repeated. Tis serial assessment to reach a diagnosis is balanced
against the rupture risk i the pregnancy is indeed ectopic. Dilation and curettage (D & C) is an option (Barnhart, 2021). It
may give a aster diagnosis but may interrupt a normal IUP.
Beore curettage, a second VS examination may be indicated
and may display new inormative ndings.
As noted, ectopic pregnancies can rupture even at low β-hCG
levels. Tus, serum β-hCG values are usually ollowed until they
lie below the negative-result threshold or the given assay.
Endometrial Findings
In a woman in whom ectopic pregnancy is suspected, VS is
perormed to look or ndings indicative o an IUP or ectopic
FiGURE 12-2 This 7-cm decidual cast was passed by a patientwith a tubal ectopic pregnancy. The cast mirrors the shape of the
endometrial cavity, and each arrow marks the portion of decidua
that lined the cornua.Ectopic Pregnancy 223
CHAPTER 12
pregnancy. During endometrial cavity evaluation, an intrauterine gestational sac is usually visible between 4½ and 5 weeks.
Te yolk sac appears between 5 and 6 weeks, and a etal pole
with cardiac activity is rst detected at 5½ to 6 weeks (Fig. 14-1,
p. 248). With transabdominal sonography, these structures are
visualized slightly later.
In contrast, with ectopic pregnancy, a trilaminar endometrial pattern is characteristic (Fig. 12-3). Its specicity is 94
percent, but with a sensitivity o only 38 percent (Hammoud,
2005). In addition, Moschos and wickler (2008) determined
in women with a PUL at presentation that no normal IUPs had
an endometrial stripe thickness <8 mm.
Anechoic uid collections, which might normally suggest
an early intrauterine gestational sac, may also be seen with
ectopic pregnancy. Tese include pseudogestational sac and
decidual cyst. First, a pseudosac is a uid collection between
the endometrial layers and conorms to the cavity shape (see
Fig. 12-3). I a pseudosac is noted, the risk o ectopic pregnancy is increased (Hill, 1990). Second, a decidual cyst is identied as an anechoic area lying within the endometrium but
remote rom the canal and oten at the endometrial–myometrial
border. Tis may represent early decidual breakdown that precedes cast ormation (Ackerman, 1993b).
Tese two ndings contrast with the intradecidual sign
seen with IUPs. With this sign, the early gestational sac is an
anechoic sac eccentrically located within one o the endometrial stripe layers (Dashesky, 1988). Te American College
o Obstetricians and Gynecologists (2020) advises caution
in diagnosing an IUP i a denite yolk sac or embryo is not
seen.
Adnexal Findings
Te sonographic diagnosis o ectopic pregnancy rests on seeing an adnexal mass separate rom the ovary (Fig. 12-4). I
an extrauterine yolk sac, embryo, or etus is identied, ectopic
FiGURE 12-3 Transvaginal sonography of a pseudogestational sac
within the endometrial cavity. Its central location is characteristic
of these anechoic fluid collections. The endometrium is marked
by calipers, and distal to this fluid, the endometrial thickness has a
trilaminar pattern. This pattern is common with ectopic pregnancy.
(Reproduced with permission from Jason McWhirt, ARDMS.)
FiGURE 12-4 Various transvaginal sonographic findings with
ectopic tubal pregnancies. For sonographic diagnosis, an ectopic
mass should be seen in the adnexa separate from the ovary and
may be seen as: (A) a yolk sac (shown here) and/or fetal pole with
or without cardiac activity within an extrauterine sac, (B) an empty
extrauterine sac with a hyperechoic ring, or (C) an inhomogeneous
adnexal mass. In this last image, color Doppler shows a classic “ring
of fire,” which reflects increased vascularity typical of ectopic pregnancies. LT OV = left ovary; SAG LT AD = sagittal left adnexal;
UT = uterus.224 First- and Second-Trimester Pregnancy Loss
Section 5
pregnancy is clearly conrmed. In other cases, a hyperechoic
halo or tubal ring surrounding an anechoic gestational sac is
seen. Alternatively, hemorrhage within the ectopic pregnancy
can orm a solid, complex adnexal mass. Overall, 60 percent
o ectopic pregnancies are a complex mass; 20 percent are a
hyperechoic ring; and 13 percent have an obvious gestational
sac with a yolk sac or embryo (Condous, 2005). Importantly,
not all adnexal masses represent an ectopic pregnancy. In this
case, integration o sonographic ndings with other clinical
inormation is necessary.
Placental blood ow within the periphery o the complex
adnexal mass—the ring o fre—can be seen with application
o color Doppler. A corpus luteum cyst oten displays a similar
vascular pattern, and dierentiation can be challenging.
Hemoperitoneum
In aected women, blood in the peritoneal cavity is most oten
identied using VS (Fig. 12-5). A small amount o peritoneal uid is physiologically normal. However, with hemoperitoneum, anechoic or hypoechoic uid initially collects
in the dependent retrouterine cul-de-sac. It then additionally
surrounds the uterus as blood lls the pelvis. With signicant
intraabdominal hemorrhage, blood will track up the pericolic
gutters to ll Morison pouch near the liver. Free uid in this
pouch typically is not seen until accumulated volumes reach
400 to 600 mL (Branney, 1995; Rodgerson, 2001). Diagnostically, peritoneal uid in conjunction with an adnexal mass and
a positive pregnancy test result are highly predictive o ectopic
pregnancy (Nyberg, 1991). Ascites rom cancer is a notable
mimic.
I sonography is unavailable, culdocentesis is a simple technique and was used commonly in the past. Te cervix is pulled
outward and upward toward the symphysis with a tenaculum,
and a long, 18-gauge needle is inserted through the posterior
vaginal ornix into the retrouterine cul-de-sac. I present, uid
can be aspirated. However, no uid is interpreted only as
unsatisactory entry into the cul-de-sac. Bloody uid or uid
with old clot ragments suggests hemoperitoneum. I the blood
sample clots, it may reect an adjacent blood vessel puncture or
brisk bleeding rom ectopic pregnancy rupture.
■ Serum Progesterone
Although not our practice, this hormone is used by some to
aid ectopic pregnancy diagnosis when serum β-hCG levels and
VS ndings are inconclusive (Stovall, 1992). A single value is
sufcient. From studies, a serum progesterone level <6 ng/mL
(<20 nmol/L) has a pooled specicity o 98 percent to predict a
nonviable pregnancy in women with a PUL (Verhaegen, 2012).
A value >25 ng/mL suggests a live IUP and excludes ectopic
pregnancy with 97-percent sensitivity (Carson, 1993). With
most ectopic pregnancies, progesterone levels range between 10
and 25 ng/mL and thus have limited diagnostic utility (American College o Obstetricians and Gynecologists, 2019c). Serum
progesterone levels can be used to buttress a clinical impression,
but again they cannot reliably identiy location (Guha, 2014).
■ Endometral Samplng
Several endometrial changes accompany ectopic pregnancy, and
all lack coexistent chorionic villi. Decidual reaction is ound in
42 percent o samples, secretory endometrium in 22 percent, and
prolierative endometrium in 12 percent (Lopez, 1994). Some
recommend that lack o chorionic villi be conrmed by D &
C beore methotrexate treatment is given. Chung and associates
(2011) ound that the presumptive diagnosis o ectopic pregnancy
is inaccurate in 27 percent o cases without histological exclusion
o a spontaneous pregnancy loss. Nevertheless, the risks o D &
C are weighed against the limited maternal risks o methotrexate.
Endometrial biopsy with a Pipelle catheter or endometrial
aspiration was studied as an alternative to surgical curettage
and ound inerior (Barnhart, 2003b; Insogna, 2017). Instead,
rozen section o curettage ragments to identiy products o
conception is accurate in 95 percent o cases (Li, 2014).
FiGURE 12-5 Hemoperitoneum. A. This transvaginal sagittal view of the pelvis shows anechoic fluid initially pooling in the retrouterine
cul-de-sac (**). Large accumulations will also extend into the anterior cul-de-sac (*). B. In this right upper quadrant sonogram, anechoic
fluid is seen in Morison pouch (arrowhead). C = cervix; F = fundus; K = kidney; L = liver. (Reproduced with permission from Dr. Devin
Macias.)Ectopic Pregnancy 225
CHAPTER 12
■ Laparoscopy
Direct visualization o the allopian tubes and pelvis by laparoscopy oers a reliable diagnosis in most cases o suspected ectopic pregnancy. Tis also permits a ready transition to denitive
operative therapy, which is discussed subsequently.
MEDiCAL MANAGEMENT
■ Regmen Optons
For most ectopic pregnancies, medical therapy is preerred, i
easible, to avoid surgical risks. Disqualiying criteria are a ruptured allopian tube and drug contraindications. Other considerations include reasonably close access to emergency care and
a commitment to surveillance laboratory testing.
Medical therapy traditionally involves the antimetabolite
methotrexate (MX). Tis drug is a olic acid antagonist. It
tightly binds to dihydroolate reductase, blocking the reduction o dihydroolate to tetrahydroolate, which is the active
orm o olic acid. As a result, de novo purine and pyrimidine
production is halted, which then arrests DNA, RNA, and protein synthesis. Tus, MX is highly eective against rapidly
prolierating trophoblast. However, gastrointestinal mucosa,
bone marrow, and respiratory epithelium also can be harmed.
o help select suitable candidates, laboratory tests are
obtained. First, MX is renally cleared, and signicant renal
dysunction, reected by an elevated serum creatinine level,
precludes its use. Second, MX can be hepato- and myelotoxic, and CBC and liver unction tests (LFs) help establish
a baseline. Last, blood type and Rh status are determined. All
except blood typing are considered surveillance laboratory tests
and are repeated prior to additional MX doses.
With administration, women are counseled to avoid several
aggravating agents until treatment is completed. Tese are: (1)
olic acid-containing supplements, which can competitively
reduce MX binding to dihydroolate reductase; (2) nonsteroidal antiinammatory drugs, which reduce renal blood ow
and delay drug excretion; (3) alcohol, which can predispose to
concurrent hepatic enzyme elevation; (4) sunlight, which can
provoke MX-related dermatitis; and (5) sexual activity, which
can rupture the ectopic pregnancy (American College o Obstetricians and Gynecologists, 2019c).
MX is a potent teratogen, and MX embryopathy is notable or cranioacial and skeletal abnormalities and etal-growth
restriction (Nurmohamed, 2011). MX is excreted into breast
milk and may accumulate in neonatal tissues and interere with
neonatal cellular metabolism (American Academy o Pediatrics, 2001; Briggs, 2017). Based on all these ndings, a list o
contraindications and pretherapy laboratory testing is ound in
Table 12-1.
For ease and efcacy, intramuscular MX administration is
used most oten or ectopic pregnancy treatment, and singledose and multidose MX protocols are available. With singledose therapy, the dose is 50 mg/m2 body surace area (BSA),
and BSA can be derived using various Internet-based BSA calculators. At our institution, patients are observed or 30 minutes ollowing MX injection to exclude an adverse reaction.
With the multidose regimen, leucovorin is added to blunt
MX toxicity. Leucovorin is olinic acid and has olic acid
activity. Tus, it allows some purine and pyrimidine synthesis
to buer side eects.
Comparing these two protocols, trade-os are recognized.
Single-dose therapy oers simplicity, less expense, and less
intensive posttherapy monitoring. However, some but not all
studies report a higher success rate or the multidose regimen
(Barnhart, 2003a; Lipscomb, 2005; abatabaii, 2012). Overall,
ectopic tubal pregnancy resolution rates approximate 90 percent
with MX use. At our institution, we use single-dose MX.
TABLE 12-1. Medical Treatment Protocols for Ectopic Pregnancy
■ Patent Selecton
Te best candidate or medical therapy is the woman who
is asymptomatic, motivated, and compliant. With medical
therapy, some classic predictors o success include a low initial
serum β-hCG level, small ectopic pregnancy size, and absent
etal cardiac activity. O these, initial serum β-hCG level is the
best prognostic indicator with single-dose MX. Reported ailure rates are 1.5 percent i the initial serum β-hCG concentration is <1000 mIU/mL; 5.6 percent at 1000 to 2000 mIU/mL;
3.8 percent at 2000 to 5000 mIU/mL; and 14.3 percent or
levels between 5000 and 10,000 mIU/mL (Menon, 2007).
Many early trials also used large size as an exclusion criterion. Lipscomb and colleagues (1998) reported a 93-percent
success rate with single-dose MX when the ectopic mass was
≤3.5 cm. Tis compared with success rates between 87 and 90
percent when the mass was >3.5 cm. Tese authors also ound
ectopic pregnancies measuring ≤4 cm and lacking cardiac
activity to be suitable candidates. Failure rates rise i cardiac
activity is seen, with an 87-percent success rate in such cases.
■ Sde Effects
Tese regimens are associated with minimal laboratory changes
and symptoms, but rarely toxicity may be severe. Kooi and
Kock (1992) reviewed 16 studies and reported that adverse
eects were resolved by 3 to 4 days ater MX was discontinued. Te most requent were liver involvement—12 percent;
stomatitis—6 percent; and gastroenteritis—1 percent. One
woman had bone marrow depression. More commonly, 65 to
75 percent o women given MX will have increasing pain
beginning several days ater therapy. Tought to reect separation o the ectopic pregnancy rom the tubal wall, this pain
generally is mild and relieved by analgesics. In a series o 258
women treated with MX by Lipscomb and colleagues (1999),
20 percent had pain that merited evaluation in a clinic or emergency department to exclude tubal rupture.
Long term, MX treatment does not diminish ovarian
reserve (Ohannessian, 2014). However, ater successul MX
therapy, pregnancy is ideally delayed or at least 3 months,
because this drug may persist in human tissues or months
ater a single dose (Hackmon, 2011). Although data are very
limited, conception beore this waiting period appears reassuring. In one study, 45 women who conceived <6 months
ater MX had similar pregnancy outcomes compared with 80
women who conceived >6 months ater MX (Svirsky, 2009).
■ Survellance
As shown in able 12-1, monitoring single-dose therapy calls
or serum β-hCG determinations at days 4 and 7 ollowing
initial MX injection on day 1. Ater single-dose MX, mean
serum β-hCG levels may rise or all during the rst 4 days
and then should gradually decline. I the level ails to drop by
≥15 percent between days 4 and 7, a second MX dose is recommended. Tis is necessary in 20 percent o women treated
with single-dose therapy (Cohen, 2014). In such cases, a CBC,
creatinine level, and LFs are rechecked. I these surveillance
tests are normal, a second equivalent dose is administered. Te
date o this second injection will become the new day 1, and
the protocol is restarted.
Multidose therapy provides MX (1 mg/kg) treatment
with leucovorin (0.1 mg/kg) therapy on alternating days. Ater
this rst pair o injections, a serum β-hCG concentration is
obtained. Values between days 1 and 3 are anticipated to drop
by ≥15 percent. I not and i surveillance tests are normal,
an additional MX/leucovorin pair is given. A serum β-hCG
level is repeated 2 days later. Up to our doses may be given i
required (Stovall, 1991).
With either dosing regimen, once a decline ≥15 percent is
achieved, weekly serum β-hCG level testing then begins until
values are undetectable. Lipscomb and colleagues (1998) used
single-dose MX to successully treat 287 women and reported
that the average time to resolution—dened as a serum β-hCG
level <15 mIU/mL—was 34 days. Te longest time was 109
days.
SURGiCAL MANAGEMENT
■ Optons
Beore surgery, uture ertility desires are discussed. In women
desiring sterilization, the unaected tube can be ligated or
removed. Tis is done concurrently with salpingectomy or the
ectopic-containing tube.
Laparoscopy is the preerred surgical approach or ectopic
pregnancy unless a woman is hemodynamically unstable. Tis
is supported rst by comparable subsequent uterine pregnancy
rates and tubal patency rates in those undergoing salpingostomy completed either by laparoscopy or by laparotomy (Hajenius, 2007). Second, laparoscopy has lower inection, adhesion,
and thromboembolism risks and aster recovery times than
laparotomy. Moreover, as experience has accrued, cases previously managed by laparotomy—or example, those with hemoperitoneum—can saely be managed laparoscopically by those
with suitable expertise. However, the lowered venous return
and cardiac output associated with pneumoperitoneum must
be actored into the selection o minimally invasive surgery or
a hypovolemic woman.
wo procedures—salpingostomy or salpingectomy—are
options. In the past, some avored salpingostomy to preserve uture ertility. However, two randomized trials compared laparoscopic outcomes between the two procedures in
women with a normal contralateral allopian tube. Te European Surgery in Ectopic Pregnancy (ESEP) study randomized 231 women to salpingectomy and 215 to salpingostomy.
Ater surgery, the subsequent cumulative rates o ongoing
pregnancy by natural conception did not dier signicantly
between groups—56 versus 61 percent, respectively (Mol,
2014). Again, in the DEMEER trial, the subsequent 2-year
rate or achieving an IUP did not dier between groups—64
versus 70 percent, respectively (Fernandez, 2013). However,
or women with an abnormal-appearing contralateral tube, salpingostomy o the ectopic-containing tube may be preerred
i easible to help preserve ertility.
O the two procedures, salpingectomy may be used or
both ruptured and unruptured ectopic pregnancies. With oneEctopic Pregnancy 227
CHAPTER 12
laparoscopic technique, the aected allopian tube is lited and
held with atraumatic grasping orceps. One o several suitable
bipolar grasping devices is placed across the allopian tube at
the uterotubal junction. Once desiccated, the tube is cut rom
its uterine attachment. Te bipolar device is then advanced
across the mesosalpinx to ree the entire tube.
Salpingostomy is typically used to remove a small unruptured pregnancy. A 10- to 15-mm linear incision is made on
the antimesenteric border o the allopian tube and over the
pregnancy. Te products usually will extrude rom the incision. Tese can be careully extracted or can be ushed out
using high-pressure irrigation that more thoroughly removes
the trophoblastic tissue. Small bleeding sites are controlled with
needlepoint electrosurgical coagulation, and the incision is let
unsutured to heal by secondary intention (ulandi, 1991).
With either procedure and ater specimen removal, the pelvis
and abdomen are irrigated and suctioned ree o blood and tissue debris to remove all trophoblastic tissue.
■ Persstent Trophoblast
Ater trophoblast removal during surgery, β-hCG levels usually
all quickly. Persistent trophoblast is rare ollowing salpingectomy but complicates 5 to 15 percent o salpingostomy cases
(Pouly, 1986; Seier, 1993). Incomplete trophoblast removal
can be identied by stable or rising β-hCG levels (Hajenius,
1995). Monitoring approaches are not codied. Weekly measures are reasonable ollowing salpingostomy (Mol, 2008).
Following uncomplicated salpingectomy, we do not repeat
β-hCG levels in women without pain or symptoms o hemoperitoneum.
With stable or increasing β-hCG levels, additional surgical or medical therapy is necessary. In those without evidence
or tubal rupture, standard therapy or persistent trophoblast is
single-dose MX, 50 mg/m2 × BSA. ubal rupture and bleeding require a second surgery.
■ Medcal versus Surgcal Therapy
O options, multidose MX treatment and laparoscopic salpingostomy have been compared in one randomized trial o 100
patients. Te authors ound no dierences or rates o tubal
preservation, primary treatment success, and subsequent ertility (Dias Pereira, 1999; Hajenius, 1997).
For single-dose MX, its efcacy compared with laparoscopic salpingostomy shows conicting results. In one randomized trial, single-dose MX was less successul in pregnancy
resolution, whereas in the other, single-dose MX was equally
eective (Saraj, 1998; Sowter, 2001). Krag Moeller and associates (2009) reported during a median surveillance period o 8.6
years that ectopic-resolution success rates and cumulative spontaneous IUP rates were not signicantly dierent between those
managed by laparoscopic salpingostomy and those treated with
single-dose MX.
Salpingectomy eectively removes the entire conceptus
and yields high resolution rates. It thus outperorms MX in
this regard. Yet, when uture ertility and ectopic pregnancy
recurrence rates are analyzed, both salpingectomy and MX
therapy show comparable results (de Bennetot, 2012; Irani,
2017). In another study, surgery, MX, or expectant management all yielded statistically similar subsequent spontaneous
IUP rates (Demirdag, 2017).
In sum, medical or surgical management oer similar outcomes in women who are hemodynamically stable, have serum
β-hCG concentrations <5000 mIU/mL, and have a small pregnancy with no cardiac activity. Despite lower success rates with
medical therapy or women with larger tubal size, higher serum
β-hCG levels, and etal cardiac activity, medical management
can be oered to the motivated woman who understands the
risks o emergency surgery in the event o treatment ailure.
■ Expectant Management
In select asymptomatic women, observation o a very early
tubal pregnancy that is associated with stable or alling serum
β-hCG levels is reasonable. A commitment to surveillance visits
and relative proximity to emergency care are other saeguards.
Importantly, this diers rom expectant management o a PUL
during its evaluation.
Predictive actors or success include a low initial serum β-
hCG concentration, a signicant drop in levels over 48 hours,
and a sonographic inhomogeneous mass rather than a tubal
halo or other gestational structures. For example, initial values <175 mIU/mL predict spontaneous resolution in 88 to 96
percent o attempts (Elson, 2004; Kirk, 2011). Initial values
<1000 mIU/mL have success rates ranging rom 71 to 92 percent (Jurkovic, 2017; Mavrelos, 2013; Silva, 2015).
With expectant management, subsequent rates o tubal
patency and intrauterine pregnancy are comparable with
surgery (Helmy, 2007). Tat said, compared with the established saety o medical or surgical therapy, the prolonged
surveillance and risks o tubal rupture support the practice
o expectant therapy only in appropriately selected and counseled women.
iNTERSTiTiAL PREGNANCY
■ Dagnoss
An interstitial pregnancy is one that implants within the tubal
segment that lies within the muscular uterine wall (Fig. 12-6).
Incorrectly, they may be called cornual pregnancies, but this
term describes a conception that develops in the rudimentary horn o a uterus with a müllerian anomaly. Risk actors
are similar to others discussed or tubal ectopic pregnancy,
although previous ipsilateral salpingectomy is a specic one
or interstitial pregnancy (Lau, 1999). Undiagnosed interstitial
pregnancies usually rupture ollowing 8 to 16 weeks o amenorrhea, which is later than or more distal tubal pregnancies. Te
myometrium covering the interstitial allopian tube segment
permits greater distention beore rupture. Because o the proximity o these pregnancies to the uterine and ovarian arteries,
hemorrhage can be severe and associated with mortality rates as
high as 2.5 percent (ulandi, 2004).
In many cases, these pregnancies are identied early, but diagnosis can still be challenging. Tese pregnancies sonographically228 First- and Second-Trimester Pregnancy Loss
Section 5
can appear similar to an eccentrically implanted IUP, especially
in a uterus with a müllerian anomaly. Criteria that may aid
dierentiation include: an empty uterus, a gestational sac seen
separate rom the endometrium and >1 cm away rom the
most lateral edge o the uterine cavity, and a thin, <5-mm
myometrial mantle surrounding the sac (imor-ritsch, 1992).
Moreover, an echogenic line, known as the interstitial line sign,
extending rom the gestational sac to the endometrial cavity
most likely represents the interstitial portion o the allopian
tube and is highly sensitive and specic (Ackerman, 1993a). In
unclear cases, three-dimensional (3-D) sonography, magnetic
resonance (MR) imaging, or diagnostic laparoscopy can help
clariy anatomy. Laparoscopically, a myometrial protuberance
is seen to lie lateral to the round ligament and coexists with
normal distal tubes and ovaries.
■ Management
Surgically, either cornual resection or cornuostomy may be per-
ormed via laparotomy or laparoscopy, depending on patient
hemodynamic stability and surgeon expertise. With either
approach, intraoperative intramyometrial vasopressin injection
may limit surgical blood loss. Cornual resection removes the
gestational sac and surrounding cornual myometrium by means
o a wedge excision (Fig. 12-7). Alternatively, cornuostomy
involves incision o the cornual myometrium and suction or
instrument extraction o the pregnancy. Both instances require
layered myometrial closure. β-hCG levels are monitored postoperatively to exclude remnant trophoblast.
With early diagnosis, medical management may be considered.
However, consensus regarding MX regimens is lacking because
o small study numbers. Jermy and coworkers (2004) reported a
94-percent success with systemic MX using a dose o 50 mg/
m2 × BSA. Others employ a traditional multidose MX regimen
(Hiersch, 2014). Direct MX injection into the gestational sac
also oers comparable success (Framarino-dei-Malatesta, 2014).
Last, a uterine artery MX inusion ollowed by uterine artery
embolization (UAE) is termed chemoembolization by some. Tis
combined with systemic MX has shown promise (Krissi, 2014).
Te risk o uterine rupture with subsequent pregnancies
ollowing either medical or surgical management is undened.
Tus, elective cesarean delivery ater 370/7 weeks’ gestation,
which is timed similarly to those with prior at-risk myomectomy, is reasonable (American College o Obstetricians and
Gynecologists, 2021).
Distinct rom interstitial pregnancy, the term angular pregnancy is used by some to describe eccentric implantation near
FiGURE 12-6 Interstitial ectopic pregnancy. A. This parasagittal view using transvaginal sonography shows an empty uterine cavity and a
mass that is cephalad and lateral to the uterine fundus (calipers). B. Intraoperative photograph during laparotomy and before cornual resection of the same ectopic pregnancy. In this frontal view, the bulging right-sided interstitial ectopic pregnancy is lateral to the round ligament
insertion and medial to the isthmic portion of the fallopian tube. (Reproduced with permission from Drs. David Rogers and Elaine Duryea.)
FiGURE 12-7 During cornual resection, the pregnancy, surrounding myometrium, and ipsilateral fallopian tube are excised en
bloc. The incision is angled inward as it is deepened. This creates a
wedge shape into the myometrium, which is then closed in layers
with delayed-absorbable suture. The serosa is closed with subcuticular style suturing. (Reproduced with permission from Hoffman
BL, Hamid CA, Corton MM: Surgeries for benign gynecologic conditions. In Hoffman BL, Schorge JO, Halvorson LM, et al: Williams
Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)Ectopic Pregnancy 229
CHAPTER 12
one cornu but within the endometrial cavity. In one prospective case series o 42 such pregnancies, 80 percent progressed to
a viable age, and abnormal placentation or uterine rupture did
not develop (Bollig, 2020). Tese eccentrically implanted early
IUPs are managed as normal pregnancies at our institution.
CESAREAN SCAR PREGNANCY
■ Dagnoss
Tis term describes implantation within the myometrium o a
prior cesarean delivery scar. Its incidence approximates 1 case
in 2000 normal pregnancies and has increased along with the
cesarean delivery rate (Rotas, 2006).
Women with symptomatic cesarean scar pregnancy (CSP)
usually present early, and pain and bleeding are common. Still,
up to 40 percent o women are asymptomatic, and the diagnosis
is made during routine sonographic examination (Rotas, 2006).
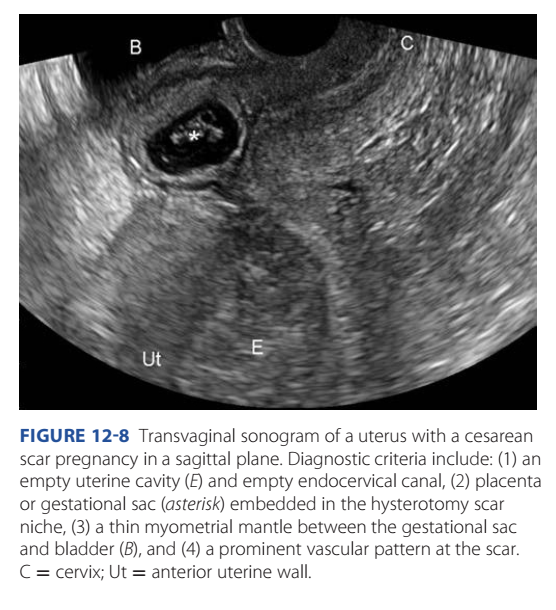
Sonographic criteria are described in Figure 12-8 (imor-
ritsch, 2012). Further, CSP implantation can be divided into
endogenic and exogenic patterns. Endogenic CSPs implant on
the scar and expand toward the uterine cavity, whereas exogenic
ones implant deeply within the scar niche and grow toward the
bladder or abdominal cavity. In one small study o CSPs that
continued to viability, endogenic CSPs yielded variable obstetric outcomes, whereas all exogenic CSPs underwent hysterectomy with placenta accreta spectrum (PAS) at delivery (Kaelin
Agten, 2017).
Sonographically, dierentiating between an IUP implanted
at the cervicoisthmic junction and a CSP can be difcult. Investigators in one study marked the midpoint o the uterine length
(cervix to undus) in sagittal views. I the center o the gestational sac lay distal to this midpoint, a CSP was diagnosed
(imor-ritsch, 2016). A spontaneous expelling abortus is
another mimic. Color Doppler will show the intense placental
vascularity around a CSP, whereas as the aborting sac is avascular. Moreover, gentle pressure applied to the cervix by the
vaginal probe will ail to move an implanted gestation—a negative sliding sign. Instead, an aborted sac will slide against the
endocervical canal (Jurkovic, 2003). VS is the typical rst-line
imaging tool, but MR imaging is useul or inconclusive cases.
■ Management
Insights into the pathogenesis o CSPs are expanding management options. Namely, growing evidence suggests that some
o these pregnancies will not behave as a typical ectopic pregnancy, and rupture rates are lower. CSPs are thought by some
to be a precursor o PAS (imor-ritsch, 2014). As such, a
signicant percentage o aected pregnancies will progress to
a viable-aged neonate, albeit with the complications associated
with PAS (Calì, 2018; imor-ritsch, 2015b).
Patients may preer to avoid rupture and PAS risks and seek
pregnancy termination. From one literature review, the most
successul operations include: (1) laparoscopic uterine isthmic
resection; (2) transvaginal isthmic resection through an anterior
colpotomy, created similarly to anterior entry during vaginal hysterectomy; (3) UAE, ollowed by D & C with or without hysteroscopy; and (4) hysteroscopic resection (Birch Petersen, 2016;
Wang, 2014). Te Society or Maternal-Fetal Medicine (SMFM)
(2020) considers sonography-guided vacuum aspiration alone, but
not sharp curettage, to be suitable. In some instances, hysterectomy
is required or may be elected in those not desiring uture ertility.
Medical management is an option or those hoping to avoid
surgery. However, compared with surgery, pregnancy resolution rates are more varied and lengthier. In one review, local
MX injection into the gestational sac alone provided a success rate o 60 percent, and systemic plus local MX raised the
rate to nearly 80 percent (Maheux-Lacroix, 2017). Te SMFM
(2020) recommends against systemic MX alone.
With local MX, doses o 1 mg/kg or 50-mg doses have been
described. Prior to local MX, etal death can be induced in
more advanced gestations by potassium chloride (KCL) injection
into the sac (Grechukhina, 2018). One option is 1 mL o 2 mEq/
mL KCL. Also, i associated bleeding complicates medical management, a Foley balloon catheter can be placed and expanded
(imor-ritsch, 2015a). Recently, a novel double-balloon catheter, in which the balloons lie in tandem, has been used with
MX to resolve CSPs (Monteagudo, 2019). Te cephalad balloon is lled within the endometrial cavity to prevent device
expulsion. Te lower balloon is tightly inated to interrupt the
CSP via mechanical pressure and tamponades potential bleeding.
Following conservative treatment, subsequent pregnancies
have good outcomes, but PAS and recurrent CSP are risks (Gao,
2016; Wang, 2015). In one series o 30 CSPs, ve subsequent
pregnancies developed normally, whereas our were recurrent
CSPs (Grechukhina, 2018). Uterine arteriovenous malormations
are a potential long-term complication (imor-ritsch, 2015b).
CSPs have also been expectantly managed. Te Society or
Maternal–Fetal Medicine (2020) recommends against this
practice. Exceptions may be early CSPs with evidence o pregnancy
ailure. One review o 69 patients continuing their gestation ound
that uterine rupture or dehiscence complicated 10 percent o all
FiGURE 12-8 Transvaginal sonogram of a uterus with a cesarean
scar pregnancy in a sagittal plane. Diagnostic criteria include: (1) an
empty uterine cavity (E) and empty endocervical canal, (2) placenta
or gestational sac (asterisk) embedded in the hysterotomy scar
niche, (3) a thin myometrial mantle between the gestational sac
and bladder (B), and (4) a prominent vascular pattern at the scar.
C = cervix; Ut = anterior uterine wall.230 First- and Second-Trimester Pregnancy Loss
Section 5
cases (Calì, 2018). During the rst or second trimester, hysterectomy was perormed in 15 percent. For the 40 patients progressing to the third trimester, 17 had placenta percreta, 23 patients
underwent hysterectomy, and two patients had uterine rupture or
dehiscence. For all trimesters, 60 percent o cases ultimately underwent hysterectomy. More reassuringly, in early pregnancies without cardiac activity, 70 percent had uncomplicated miscarriage,
whereas 30 percent required surgical or medical intervention. O
these early demises, none required hysterectomy.
Women accepting expectant care are ideally well counseled
on these potential obstetric complications. I not prompted by
earlier complications, repeat cesarean delivery is recommended
at 340/7 and 356/7 weeks’ gestation, and this timing recognizes
the PAS and uterine rupture risks associated with CSP. Betamethasone to hasten lung maturity is recommended prior to
delivery (Society or Maternal–Fetal Medicine, 2020).
CERViCAL PREGNANCY
■ Dagnoss
Tis rare ectopic pregnancy is dened rst by cervical glands
noted histologically opposite the placental attachment site. Second, all or part o the placenta lies at a level below the entrance
o the uterine vessels or below the peritoneal reection on the
anterior uterus. rophoblast invades the endocervix, and the
pregnancy develops in the brous cervical wall. Risk actors
include AR and prior uterine curettage (Ginsburg, 1994).
Painless vaginal bleeding is reported by 90 percent o women
with a cervical pregnancy, and it can be severe (Ushakov,
1997). As pregnancy progresses, a distended, thin-walled cervix
with a partially dilated external os may be evident. Above the
cervical mass, a slightly enlarged uterine undus is elt. ypical
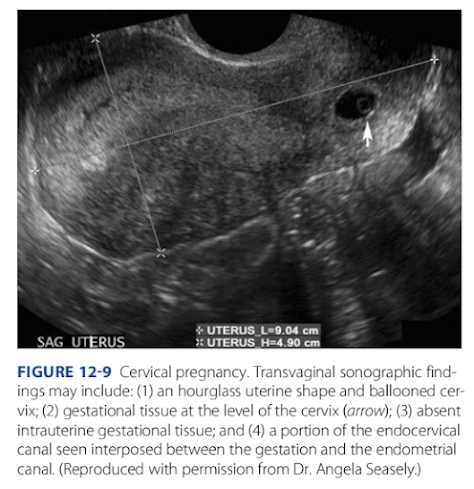
sonographic ndings are shown and described in Figure 12-9.
In some cases, MR imaging and 3-D VS can aid diagnosis.
At times, cervical ectopic pregnancy can mimic a miscarriage
in transit through the cervix. Similar to CSPs and described in
that section, color Doppler will show the intense vascularity o
cervical implantation, and gentle pressure applied to the cervix
by the vaginal probe will ail to move an implanted gestation—
a negative sliding sign.
■ Management
Cervical pregnancy may be treated medically or surgically.
In many centers, including ours, MX is rst-line therapy in
hemodynamically stable women. O options, single- or multidose systemic MX and dosing ound in able 12-1 are
suitable (Murji, 2015). Alternatively, 50 mg o MX can be
injected directly into the gestational sac (Jeng, 2007; Yamaguchi, 2017). Others describe chemoembolization with MX and
UAE, as described or interstitial pregnancy (p. 228).
With MX regimens, resolution and uterine preservation are achieved or gestations <12 weeks in 91 percent
o cases (Kung, 1997). o select appropriate candidates,
Hung and colleagues (1996) noted higher risks o systemic
MX treatment ailure in those with a gestational age >9
weeks, β-hCG levels >10,000 mIU/mL, crown-rump length
>10 mm, and etal cardiac activity. For this reason, eticidal KCL can be injected into the etus or gestational sac
(Verma, 2009). Notably, during posttherapy surveillance,
sonographic resolution lags ar behind serum β-hCG level
regression (Song, 2009).
Although conservative management is easible or many
women with cervical pregnancies, suction evacuation or hysterectomy may be selected. Moreover, hysterectomy may be
required with bleeding uncontrolled by conservative methods
(Fowler, 2021). During hysterectomy, because o the close proximity o the ureters to the ballooned cervix, urinary tract injury
rates are a concern.
I suction evacuation o the cervix is planned, intraoperative
bleeding may be lessened by preoperative UAE, by intracervical vasopressin injection, or by a cerclage placed at the internal
cervical os to compress eeding vessels (Chen, 2015; Fylstra,
2014; Wang, 2011). Cervical branches o the uterine artery
can eectively be ligated with vaginal placement o hemostatic
cervical sutures on the lateral aspects o the cervix at 3 and
9 o’clock (Bianchi, 2011).
As an adjunct to medical or surgical therapy, UAE has been
described either as a response to bleeding or as a preprocedural
prevention (Hirakawa, 2009; Zakaria, 2011). Also, in the event
o hemorrhage, a 26F Foley catheter with a 30-mL balloon can
be placed intracervically and inated to eect hemostasis by
vessel tamponade and to monitor bloody drainage. Te balloon
remains inated or 24 to 48 hours and is gradually decompressed over a ew days (Ushakov, 1997).
ABDOMiNAL PREGNANCY
■ Dagnoss
Tese rare ectopic pregnancies are dened as an implantation in the peritoneal cavity exclusive o tubal, ovarian, or
FiGURE 12-9 Cervical pregnancy. Transvaginal sonographic findings may include: (1) an hourglass uterine shape and ballooned cervix; (2) gestational tissue at the level of the cervix (arrow); (3) absent
intrauterine gestational tissue; and (4) a portion of the endocervical
canal seen interposed between the gestation and the endometrial
canal. (Reproduced with permission from Dr. Angela Seasely.)Ectopic Pregnancy 231
CHAPTER 12
intraligamentous implantations. Most are thought to ollow
early tubal rupture or tubal abortion with reimplantation.
Clinically, symptoms may be absent or vague. Laboratory
tests are typically uninormative, although maternal serum
alpha-etoprotein levels can be elevated. With later gestations,
abnormal etal positions may be palpated, or the cervix is displaced (Zeck, 2007). Sonographically, clues are a etus or placenta seen eccentrically positioned within the pelvis or separate
rom the uterus; lack o myometrium between the etus and
the maternal anterior abdominal wall or bladder; or bowel
loops surrounding the gestational sac (Allibone, 1981; Chukus,
2015). Oligohydramnios is common but nonspecic. Oten
needed, MR imaging can aid diagnosis and provide placental
inormation.
■ Management
Abdominal pregnancy treatment depends on the gestational
age at diagnosis. Conservative expectant management carries
a maternal risk or sudden, dangerous hemorrhage. Moreover,
Stevens (1993) reported etal malormations and deormations in 20 percent. Tus, we believe that termination generally is indicated once the diagnosis is made. Certainly, beore
24 weeks’ gestation, conservative treatment rarely is justied.
Despite this, some describe waiting until etal viability with
close surveillance (Harirah, 2016).
Principal surgical objectives are delivery o the etus and
careul assessment o placental implantation without provoking hemorrhage. Unnecessary exploration is avoided because
the anatomy is commonly distorted and surrounding areas are
extremely vascular. Importantly, placental removal may precipitate torrential hemorrhage because the normal hemostatic
mechanism o myometrial contraction to constrict hypertrophied blood vessels is lacking. I it is obvious that the placenta
can be saely removed or i the implantation site is already
bleeding, then removal begins immediately. Blood vessels supplying the placenta are ideally ligated rst. For early gestations,
locally injected dilute vasopressin also can be employed.
Some advocate leaving the placenta in place as the lesser o
two evils. It decreases the chance o immediate lie-threatening
hemorrhage, but at the expense o long-term sequelae. Placental embolization may play a role prior to or ollowing etal
extraction (Frischhertz, 2019; Marcelin, 2018). I let in the
abdominal cavity, the placenta can orm abscesses, adhesions,
intestinal or ureteral obstruction, and wound dehiscence (Bergstrom, 1998; Martin, 1988). In many o these cases, surgical
removal becomes inevitable. I the placenta is let, its involution can be monitored using serum β-hCG levels and color
Doppler sonography or MR imaging (France, 1980; Martin,
1990). Placental unction usually declines rapidly. Te placenta
is eventually resorbed, but this can take months or years with
advanced gestations (Valenzano, 2003).
I the placenta is let, postoperative MX is oten given to
hasten involution. Accelerated placental destruction with accumulation o necrotic tissue ollows (Deng, 2017). Inection
with abscess ormation can be a complication (Rahman, 1982).
Similar to persistent trophoblastic tissue, early gestations may
benet most (Ansong, 2019).
OVARiAN PREGNANCY
Ectopic implantation o the ertilized egg in the ovary is rare
and is diagnosed i our clinical criteria are met. Tese were
outlined by Spiegelberg (1878): (1) the ipsilateral tube is intact
and distinct rom the ovary; (2) the ectopic pregnancy occupies
the ovary; (3) the ectopic pregnancy is connected by the uteroovarian ligament to the uterus; and (4) ovarian tissue can be
demonstrated histologically amid the placental tissue. Risk actors are similar to those or tubal pregnancies, but AR or IUD
ailure are prominent (Zhu, 2014). Presenting complaints and
ndings mirror those or tubal ectopic pregnancy. Although the
ovary can accommodate the expanding pregnancy more easily
than the allopian tube, rupture at an early stage is the usual
consequence (Melcer, 2016).
Sonographically, an internal anechoic area is surrounded by a
wide echogenic ring, which in turn is surrounded by ovarian cortex (Comstock, 2005). In one review o 49 cases, the diagnosis
was not be made until surgery, and many cases were presumed to
be a tubal ectopic pregnancy (Choi, 2011). Moreover, at surgery,
an early, unrecognized ovarian pregnancy may instead be considered and managed as a hemorrhagic corpus luteum.
Evidence-based management accrues mainly rom case
reports (Hassan, 2012). Classically, management or ovarian
pregnancies has been surgical. Selection o laparoscopy or laparotomy are inuenced by gestational age, hemoperitoneum,
and hemodynamic status. Small lesions can be managed by
ovarian wedge resection or cystectomy, whereas larger lesions
require oophorectomy (Elwell, 2015; Melcer, 2015). With
conservative surgery, β-hCG levels should be monitored to
exclude remnant trophoblast.
HETEROTOPiC PREGNANCY
Tis pairing o an IUP and an ectopically located pregnancy
is rare, and the most common dyad is an IUP and an ampullary tubal pregnancy. Te natural incidence o these heterotopic
pregnancies approximates 1 case per 30,000 pregnancies (Reece,
1983). However, with AR, their incidence is higher and is
9 cases in 10,000 pregnancies (Perkins, 2015). Initial clinical
symptoms usually reect those rom the ectopic. Because an
IUP is seen sonographically and the ectopic pregnancy may not
be visualized, rates o rupture are higher in heterotopic pregnancy (Dendas, 2017).
In patients wishing to preserve the IUP, management initially is dictated by bleeding. In those with hemorrhage, treatment o the ectopic pregnancy is surgical. Depending on the
ectopic location, resection or suction aspiration is the most
common method (Wu, 2018). O note, adjunctive UAE and
vasopressin and their eects on uterine blood ow are less
desirable or the ongoing IUP. With a rare comorbid ovarian
ectopic pregnancy, early excision o the corpus luteum merits
progesterone supplementation (Chap. 66, p. 1170).
In those without signicant bleeding, medical steps to disrupt the ectopic pregnancy typically involve gestational-sac
injection o KCl or o hyperosmolar glucose. Tis may be ollowed by later aspiration evacuation o the ectopic gestation.
Because o toxicity to the IUP, MX is avoided.232 First- and Second-Trimester Pregnancy Loss
Section 5
With ongoing pregnancy, adverse neonatal outcome rates are
not appreciably elevated (Clayton, 2007). However, initial sonographic surveillance o etal growth seems reasonable. Route o
ultimate delivery is inuenced mainly by myometrial integrity
ollowing ectopic treatment (Dendas, 2017; OuYang, 2014).








Nhận xét
Đăng nhận xét