Chapter 12 Surgery for Ovarian and Peritoneal Disease
GENERAL PRINCIPLES
Definition
■ Endometriosis is a chronic, nonmalignant, condition characterized by pelvic pain and infertility that is hormonally mediated and responsive to hormone suppression and surgical excision. It is defined by the histologic identification of ectopic implants of endometrial glands and stroma. These implants are commonly found on the pelvic peritoneum, abdominal, and pelvic organs. Endometriosis can also be found within remote locations such as the thoracic cavity. The pathogenesis of endometriosis remains unclear.
Differential Diagnosis
■ Adhesions, pelvic inflammatory disease, mittelschmerz, chronic pelvic pain, malignancy, hemorrhagic ovarian cyst.
Nonoperative Management
■ Unfortunately, the only way to diagnose endometriosis is with tissue biopsies obtained during surgery. Once surgical pathology confirms ectopic endometriosis tissue, conservative nonoperative management with oral contraceptive pills and nonsteroidal anti-inflammatory drugs is recommended to inhibit ovulation and decrease pain. Other medications such as progestins, gonadotropin-releasing hormone agonists, and aromatase inhibitors may also be utilized to suppress the disease.
Nonoperative medical management of this chronic disease is a cornerstone of treatment and should accompany surgical management.
Figure 12.1. 5.5-cm endometrioma identified by TVUS.
IMAGING AND OTHER DIAGNOSTICS
Occasionally a speculum examination can reveal vaginal endometriosis implants that can be biopsied and confirm the diagnosis. More commonly, the pelvic examination yields suggestive but nonspecific findings of endometriosis such as decreased uterine mobility, a palpable adnexal mass, or rectovaginal and uterosacral nodules.
■ Transvaginal ultrasound is the diagnostic imaging modality of choice for identifying ovarian endometriomas (Fig. 12.1). Rectal endometriosis can also be seen with transvaginal ultrasound, particularly with the addition of rectal contrast, and requires experienced sonographers and a high level of radiographic expertise.
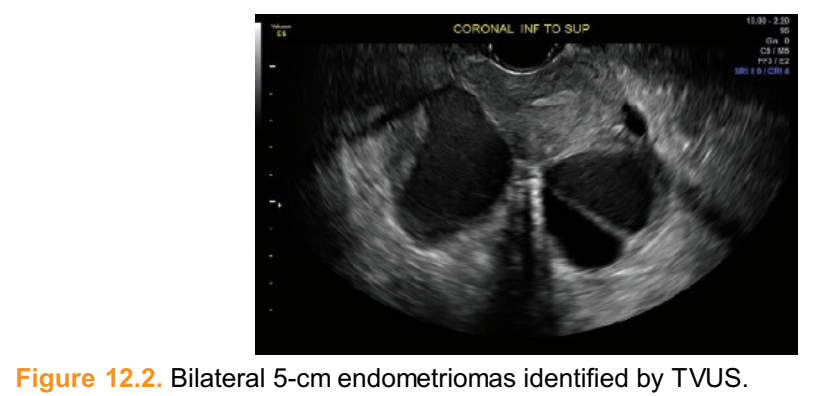
■ Small endometriomas are identified by abdominal or vaginal ultrasounds obtained at least 6 to 8 weeks apart to differentiate them from hemorrhagic corpus luteal cysts that usually involute during this time period. Larger endometriomas that are 4 to 5 cm or more in diameter are usually diagnosed by their characteristic homogeneous pattern (Fig. 12.2). The hypoechoic cyst may contain diffuse low-level echoes with septations and multiloculations and may not benefit from repeat imaging.
■ Magnetic resonance imaging (MRI), with enterography, is reserved for equivocal ultrasound findings or for patients with a clinical history consistent with deep infiltrating endometriosis invading the bowel or bladder.
■ Computed tomography (CT) is not a recommended imaging modality.
■ For patients who desire fertility, have struggled with infertility, are 35 years of age or older, or have ovarian endometriomas, a serum antimüllerian hormone level may be useful for fertility counseling.Figure 12.2. Bilateral 5-cm endometriomas identified by TVUS.
■ Currently, there are no reliable serum markers for endometriosis. Ca-125 can be elevated in endometriosis and is not recommended unless there is a strong suspicion for malignancy.
PREOPERATIVE PLANNING
■ An examination under anesthesia is performed to assess uterine position, sacral nodularity, and palpable adnexal masses that may affect incision length or laparoscopic trocar placement.
■ Angled 30- or 45-degree laparoscopes can increase the range of surgical views, particularly for large adnexal masses.
■ Cystoscopy is indicated when extensive sidewall adhesiolysis or ureterolysis is performed. Intraoperative intravenous indigo carmine or 10% sodium fluorescein, or presurgical administration of oral pyridium provides urine contrast for easy identification of the urine jets from the ureteral orifices.
SURGICAL MANAGEMENT
■ Indications for surgical intervention may include the need to obtain tissue diagnosis, pain refractory to medical management, contraindications to medical therapy, to resect deep infiltrating endometriosis that is causing obstruction to the genitourinary or gastrointestinal tracts, to exclude malignancy in an adnexal mass, to improve pregnancy rates in infertile patients with suspected endometrioma, and to treat chronic pain in the infertile patient who desires pregnancy.
■ Although endometriosis historically shares many characteristics that are similar to malignancy (tissue biopsy diagnosis, surgically staged condition, and it is colloquially referred to as recurrence of disease instead of persistence of disease) it is crucially important to remember that endometriosis is a benign and chronic condition. Application of malignant surgical principles such as debulking and cytoreduction tend to supersede the overall well-being of the patient. Surgical interventions forendometriosis must be thoughtful and tempered. Extreme surgical management that results in significant patient morbidity or decreased functionality is not encouraged.
■ When endometriosis affects areas that are vulnerable to tissue damage and destruction, such as ovarian follicles, ablative techniques may be appropriate.
■ Optimal endometrioma treatment in reproductive age women weighs inadvertent follicle destruction against endometrioma recurrence.
■ Current endometrioma surgical management is largely influenced by a Cochrane review that demonstrated endometriomas greater than 3 cm have a higher recurrence rate when ablated with bipolar energy versus cyst excision.
■ Recent investigations suggest that cyst fenestration and plasma vaporization may preserve antral follicles without compromising cyst recurrence or subsequent pregnancy rates.
■ While we await randomized, prospective investigation that confirms this claim, surgeons must reconsider their techniques to balance adequate treatment of symptomatic disease against unintentional reduction of the very fertility they wish to preserve.
■ A key to both endometrioma and peritoneal endometriosis excision is identification and separation of endometriosis from healthy tissue.
■ The goal of the surgery is paramount and should dictate the degree of surgical intervention. For some patients, excision of a pelvic lesion to exclude malignancy is sufficient. For others, restoration of anatomy and resection of deep infiltrating disease are necessary.
Positioning
■ Please see low lithotomy positioning described in Chapter 5.
■ Laparoscopy: The patient’s arms are gently tucked and extremities protected with padding. Her legs comfortably rest in neutral position in adjustable leg stirrups that allow for perineal access and manipulation of the uterus.
Approach
A minimally invasive, laparoscopic approach is the preferred surgical approach for this benign disease. Extensive adhesiolysis, excision, and ablation can be performed safely by an experienced laparoscopic surgeon.Endometrioma Excision (Video 12.1)
Identify anatomic landmarks
■ First, perform an initial survey to identify landmarks—this is especially helpful in the case of distorted anatomy.
■ The bilateral medial and lateral umbilical ligaments are helpful to orient the anatomic space, to ensure safe dissection (Tech Fig. 12.1A–C). Perform adhesiolysis and expose the ureter.
Tech Figure 12.1. A: The medial umbilical ligament contains the obliterated umbilical artery and points to the anterior division of the internal iliac artery. The lateral umbilical fold contains the inferior epigastric vessels. B: Distorted anatomy endometrioma right tube, and bowel. C: Endometrioma, myoma, left adnexa.
Incise the thinnest area of ovarian cortex overlying the endometrioma
■ See Tech Figure 12.2.Tech Figure 12.2. Endometrioma with fibrotic interface.
Perform adhesiolysis of the fibrotic interface from the ovarian cortex and if necessary decompress the cyst
■ The characteristic expulsion of old heme or “chocolate fluid” signals entry into the endometrioma and the contents are evacuated and the cyst is decompressed (Tech Fig. 12.3A,B).Tech Figure 12.3. A: Identify and incise the thin ovarian cortex, exposing the endometrioma. B: Evacuate and decompress the cyst.
Identify and bluntly separate the endometrioma from the ovarian parenchyma
■ To reduce follicle destruction, meticulous, controlled, traction and countertraction are applied to the opposing tissue planes (Tech Fig. 12.4A). Large endometriomas will be turned inside out with progressive dissection.
■ Forceful tissue separation causes “stripping” of ovarian follicles, therefore, thisshould be avoided.
■ The endometrioma cyst wall will appear thick and white and adherent to ovarian tissue. Once in the correct cleavage plane, the cyst wall will easily yield and separate from ovarian parenchyma in bursts with blunt dissection (Tech Fig. 12.4B,C).
Tech Figure 12.4. A: Identify and bluntly separate the endometrioma from the ovarian parenchyma. B: Endometrioma cyst wall will be thick, white, and moderately adherent to ovary. C: Apply countertraction and gently sweep along the cleavage plane to separate the tissue.
Vaporize obstructing adhesions
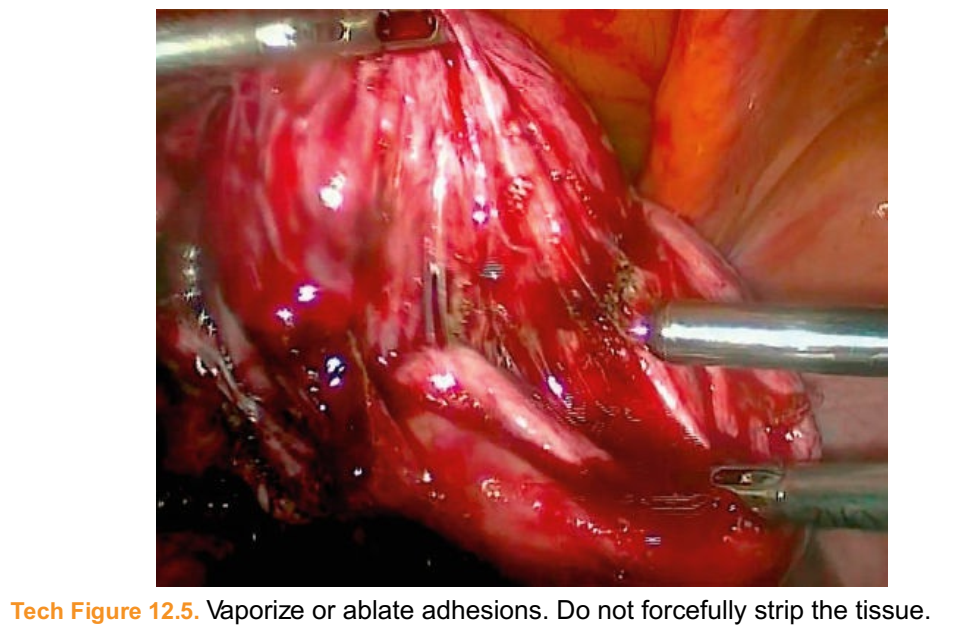
■ Once this technique no longer advances tissue separation, plasma energy can be employed to vaporize the obstructing adhesions (Tech Fig. 12.5).
■ Hold the handpiece 2 to 3 mm away and perpendicular to the adhesion.
■ Continue to excise the cyst wall using these principles.
■ Apply sparse bipolar energy to fibrotic perihilar adhesions and incise with scissors.Tech Figure 12.5. Vaporize or ablate adhesions. Do not forcefully strip the tissue.
Achieve hemostasis
■ Coagulate only active bleeding and resist the urge to “seal” the surface of the ovarian parenchyma (Tech Fig. 12.6).
■ Hold the handpiece approximately 8 to 10 mm away and perpendicular to the tissue.
■ The area adjacent to the hilum contains numerous vessels.
■ Employ bipolar energy for vessels.Tech Figure 12.6. Achieve hemostasis.
Remove the cyst wall, irrigate and perform final survey (Tech Fig. 12.7)
Tech Figure 12.7. Irrigate and perform final inspection.Peritoneal Endometriosis Excision
Identify anatomic landmarks
■ Locate vessels and ureters and note their distance from the target pathology. Perform adhesiolysis and ureterolysis. Evaluate the extent of endometriosis invasion. Grasp the peritoneal endometriosis and apply traction
■ Initially grasp only one thin cell layer of peritoneum. Incise the peritoneum with either laparoscopic scissors, radiofrequency energy, or plasma energy
■ Allow the pneumoperitoneum to dissect the tissue plane (Tech Fig. 12.8A,B).
Tech Figure 12.8. A: Incise the peritoneum. B: Allow the pneumoperitoneum to separate the tissue.
Regrasp the tissue and apply traction away from the sidewall, leaving the area of deepest infiltration/nodule until the end
■ To facilitate dissection, an assistant may apply countertraction with a laparoscopic grasper (Tech Fig. 12.9A,B).
Tech Figure 12.9. A: Apply traction away from the pelvic sidewall. B: To facilitate dissection, apply countertraction with a laparoscopic grasper.
Once isolated, grasp the nodule to expose the deeper fibrotic attachments
■ Excise the lesion from the surrounding healthy tissue.
■ These attachments may range from cement-like concretions to sticky peritoneal whisps. Obtain hemostasis using sparse radiofrequency energy, plasma energy, or nonthermal hemostatic agent
PEARLS AND PITFALLS
Review pelvic anatomy vasculature in relation to ligaments and peritoneal folds. An understanding of vital structures and their usual relationships will aid in safe dissection when faced with severely distorted anatomy. For example:
• Apply traction to the round ligament to identify the deep inguinal ring. Just medial and anterior to this insertion are the deep inferior epigastric vessels (contained within the lateral umbilical fold) that arise from the external iliac artery.
• Follow the medial umbilical ligament past its intersection with the round ligament as it points toward the anterior division of the internal iliac artery.
Do not rely solely on the uterus and adnexa as anatomic landmarks.
Identify the median, medial, and umbilical ligaments to frame the pelvis. Then, identify the ureter and vasculature.
Identify and avoid incising the ovarium hilum. Premature entry into this vascular area will result in heavy and early blood loss that will complicate endometrioma excision.
Abrupt brisk bleeding obscures the visual field, prolongs surgery, and compromises patient well-being. Therefore, immediately achieve hemostasis with plasma surface sealing or bipolar energy.
Grasp the peritoneal tissue to apply tissue traction. With an opposing blunt instrument, apply countertraction in a unidirectional, gentle sweeping motion to allow blunt dissection along natural cleavage planes which decreases blood loss.
Avoid hydrodissection of the peritoneal tissue as it obscures tissue planes and hampers tissue excision.
After initial incision, allow the pneumoperitoneum to “poof up” the unscarred peritoneal tissue which creates an avascular space of areolar tissue.
POSTOPERATIVE CARE
■ The majority of endometrioma and peritoneal endometriosis excision procedures may be performed as outpatient surgery. Postoperative care is similar to any other laparoscopic surgery. Immediate postoperative analgesia should include narcotic pain relief with concomitant stool softener and nonsteroidal anti-inflammatory drugs.
■ All patients should receive secondary prevention, postoperative drug therapy to decrease disease persistence or recurrence. A myriad of oral contraceptive pills, progestins, GnRH agonists, aromatase inhibitors, Danazol, and hormone-secreting IUDS are available for this adjuvant medical therapy.
OUTCOME
■ Incision and drainage of endometriomas has been abandoned due to endometrioma reformation. Endometrioma excision is currently the favored approach because of two randomized studies that revealed laparoscopic endometrioma excision was associated with a reduced recurrence rate of dysmenorrhea, dyspareunia, nonmenstrual pelvic pain, endometrioma recurrence and with a reduced requirement for further surgery compared to endometrioma ablation alone.
■ Furthermore, for women attempting to conceive there was an increase in spontaneous pregnancy rate for those who had documented preoperative subfertility with endometrioma excision.
■ Endometrioma ablation with novel plasma energy is promising but the results of a randomized study are still pending and further research is required.
■ Endometrioma recurrence has been reported to be between 6% and 17%. Postoperatively, patients who took continuous oral contraceptive pills reduced endometrioma recurrence from 29% to 8% while those who used cyclic oral contraceptives reduced recurrence to only 15%.
COMPLICATIONS
■ Inadvertent ovarian tissue destruction underscores the importance of surgical technique and expertise. Pathology specimens confirm that normal ovarian tissue is commonly removed with the endometrioma wall and the larger the cyst diameter, the more normal ovarian tissue is removed.
■ Control brisk bleeding with direct pressure, suture ligation, vascular clips, and bipolar energy as needed. Large arterial bleeding cannot be controlled by topical hemostasis agents alone and heavy bleeding may necessitate oophorectomy if attempts to control bleeding are unsuccessful.
■ Nondiscrete bleeding of friable tissue can be achieved with topical hemostatic agents such as oxidized regenerated cellulose, thrombin, fibrin sealants, and microporous polysaccharide spheres. Caution and judicious use of these products is advised since some of these products have been implicated with causing widespread pelvic inflammation and bowel obstruction.
■ Ureter injury may be recognized in the immediate postoperative period or may take up to 14 days to become apparent if due to thermal spread. Proficiency in ureterolysis is essential in order to safeguard the ureter and to avoid puncture and thermal injury. Intraoperative cystoscopy may provide some reassurance of ureteral integrity and allow for bladder evaluation.
■ Thermal bowel injury is usually apparent by postoperative days 3 to 5. Route of repeat surgery depends on the patient’s clinical condition butlaparotomy may be required to optimally decrease contamination and to avoid further complications.









Nhận xét
Đăng nhận xét