Vaginal Delivery
BS. Nguyễn Hồng Anh
The natural culmination of second-stage labor is controlled vaginal delivery of a healthy neonate with minimal trauma to the mother. Although some clinical settings favor cesarean delivery, for most fetuses, vaginal birth is preferred. For the mother, spontaneous vaginal vertex delivery poses the lowest risk of most maternal comorbidity, and comparisons with cesarean delivery are found in Chapter 30 (p. 548). Delivery is usually spontaneous, although some maternal or fetal complications may warrant operative vaginal delivery, described in Chapter 29 (p. 533).
Last, a malpresenting fetus or multifetal gestation in many cases may be delivered vaginally but requires special techniques. These are described in Chapters 28 and 48.
DELIVERY TECHNIQUE
■ Preparation
The end of second-stage labor is heralded as the perineum begins to bulge, and the fetal scalp is seen through the separating labia. Perineal pressure from the fetal head creates reflexive pushing efforts. At this time, additional staff to attend the neonate and instruments are readied for delivery. Fetal heart rate monitoring continues. As one example, a nuchal cord often tightens with fetal descent and may lead to deepening variable decelerations. If the bladder is distended, catheterization can provide added pelvic space.
During second-stage labor, pushing positions may vary. But for delivery, the dorsal lithotomy position is most common and often the most satisfactory. Leg holders or stirrups are often used to assist. With or without their use, perineal laceration rates were equal in one randomized study of 214 parturients (Corton, 2012). With positioning, legs are not separated too widely or placed one higher than the other. Legs are not strapped into the stirrups. This permits quick flexion of the thighs backward onto the abdomen should shoulder dystocia develop. Legs may cramp during second-stage pushing, and cramping can be relieved by creating gentle muscle stretch, by brief massage, or both.
Preparation for delivery includes vulvar and perineal cleansing. If desired, sterile drapes are placed to cover the legs and abdomen and expose only the perineum. Scrubbing, gowning, gloving, and donning protective mask and eyewear protect both the gravida and accoucheur from infectious agents.
■ Delivery of the Head
By the end of second-stage labor, the position of the occiput is usually known. In some cases, however, molding and caput formation may have precluded early accurate identification. At this time, careful assessment is again performed as described in Chapter 22 (p. 429). In most cases, position is occiput anterior (OA) or is rotated slightly oblique. But, in perhaps 5 percent, an occiput posterior (OP) position persists. With each contraction, the vulvovaginal opening is dilated by the fetal head to gradually form an ovoid and finally, an almost circular opening. This encirclement of the largest head diameter by the vulvar ring is termed crowning. The anus becomes greatly stretched, and the anterior wall of the rectum is easily seen through it. The perineum thins and may spontaneously lacerate. Third- and fourth- degree perineal lacerations, which by definition involve the anal sphincter and perineal body, are collectively termed obstetric anal sphincter injuries (OASIS). Because of their associated morbidity (p. 508), one main goal during delivery is OASIS prevention. To improve perineal elasticity and protect against laceration, some perform daily antepartum perineal massage starting at 34 to 35 weeks’ gestation. With a lubricant, the woman or her partner inserts one or two fingers 3 cm into the vagina and applies pressure, first downward for 2 minutes and then laterally to each side of the vaginal entrance for 2 minutes. In one large randomized study, nulliparas benefited mainly by lower rates of episiotomy and of first-degree laceration (Labreque, 1999). Third- and fourth-degree laceration rates are not lowered (Beckmann, 2013). As another tool, antepartum use of the Epi-No intravaginal pump balloon similarly aims to stretch the perineum. However, in randomized trials, it did not prevent episiotomy, perineal trauma, or levator injury (Brito, 2015; Kamisan Atan, 2016). Instead of antepartum use, perineal massage can be performed solely intrapartum during second-stage labor. In a large randomized study, the rates of episiotomy, OASIS, and intact perineum were similar to those in a nonmassage group (Stamp, 2001). In subsequent systematic reviews, this practice was noted to lower OASIS rates and raise rates of delivery over an intact perineum (Aasheim, 2017; Aquino, 2020a).
When the head distends the vulva and perineum enough to open the vaginal introitus to a diameter of 5 cm or more, slow delivery of the head and control of accelerative forces may minimize lacerations (Laine, 2008). One of two approaches may be instituted. With the hands-on method, the thumb and remaining fingers form a V pressed against the perineum to bolster it. The other hand maintains fetal neck flexion to deliver the smallest head diameter through the introitus and provides gentle pressure against rapid forward movement to avoid expulsive delivery. Instead, with the hands-poised method, hands touch neither the head or perineum but are applied only selectively to slow rapid head expulsion. In a large randomized trial with more than 5000 gravidas, rates of intact perineum or OASIS did not differ between groups. In the hands-poised cohort, episiotomy rates were lower (McCandlish, 1998). Subsequent systematic reviews of randomized trials similarly note lower episiotomy rates with a hands-poised approach. However, among these analyses, intact perineum or OASIS rates were not consistently benefited by this method (Aasheim, 2017; Huang, 2020; Pierce-Williams, 2019).
Alternatively, if expulsive efforts are inadequate or an expedited delivery is needed, the Ritgen maneuver may be employed or an episiotomy cut. With the maneuver, gloved fingers grasp the fetal chin just behind or in the anus and exert upward pressure between contractions. As a counter, the other hand presses against the occiput to control forces against the perineum (Fig. 27-1) (Cunningham, 2008). Although expeditious, this maneuver does not alter OASIS rates (Aquino, 2020b).
FIGURE 27-1 Modified Ritgen maneuver. Moderate upward pressure is applied to the fetal chin by the posterior hand covered with a sterile towel, while the occiput is held against the symphysis.■ Delivery of the Shoulders
Following delivery of the fetal head, a finger is passed across the fetal neck to determine whether it is encircled by one or more umbilical cord loops. The nuchal cord incidence increases with gestational age and is found in nearly 25 percent of deliveries at term (Larson, 1997). If an umbilical cord coil is felt, it is slipped over the head if loose enough. If applied too tightly, the loop is cut between two clamps. \Tight nuchal cords complicate approximately 6 percent of all deliveries but are not associated with worse neonatal outcomes than those without a cord loop (Henry, 2013).
Following its delivery, the fetal head falls posteriorly, bringing the face almost into contact with the maternal anus. The occiput promptly turns toward one of the maternal thighs, and the head assumes a transverse position. This external rotation indicates that the bisacromial diameter, which is the distance between the shoulders, has rotated into the anteroposterior diameter of the pelvis.
Most often, the shoulders appear at the vulva just after external rotation and are born spontaneously. If delayed, extraction aids controlled delivery. The sides of the head and neck are grasped with two hands, and if needed, gentle axial traction may be applied until the anterior shoulder appears under the pubic arch. Axial traction is aligned with the fetal spine, and lateral deviation of the neck does not exceed 25 to 45 degrees (American College of Obstetricians and Gynecologists, 2019c). Next, the posterior shoulder is delivered. During delivery, abrupt or powerful lateral extension of the neck is avoided to avert brachial plexus injury.
The rest of the body almost always follows the shoulders without difficulty. With prolonged delay, however, its birth may be hastened by moderate outward traction on the head and moderate pressure on the uterine fundus. Hooking the fingers in the axillae is avoided. This can injure upper extremity nerves and produce a transient or possibly permanent paralysis. After the newborn, a gush of amnionic fluid that can be blood-tinged but not grossly bloody usually follows. Previously, immediate nasopharyngeal bulb suctioning of the newborn was routine to remove secretions. However, suctioning of the nasopharynx may lead to neonatal bradycardia (Gungor, 2006). American Heart Association neonatal resuscitation recommendations eschew most suctioning immediately following birth—even with meconium present (Chap. 32, p. 589). Moreover, with meconium-stained fluid, routine intubation for tracheal suction is not recommended for vigorous or for nonvigorous neonates. Suctioning is reserved for neonates who have obvious obstruction to spontaneous breathing or who require positive-pressure ventilation (Wycko, 2015). For suctioning, options are bulb syringe or suction catheter aspiration and may include intubation and suctioning if the airway is obstructed.
■ Cord Clamping
The umbilical cord is cut between two clamps placed 6 to 8 cm from the fetal abdomen, and later an umbilical cord clamp is applied 2 to 3 cm from its insertion into the fetal abdomen. For vigorous term neonates, umbilical cord clamping is ideally delayed 30 to 60 seconds to transfer blood to the newborn. Resting the newborn below the level of the uterus allows gravity to aid flow (Yao, 1974). As a result, higher total body iron stores and lower anemia rates for the neonate are sustained (Andersson, 2011; McDonald, 2013). This practice may be particularly valuable in populations in which iron deficiency is prevalent (World Health Organization, 2014). In general, delayed compared with early umbilical cord clamping does not worsen Apgar scores or umbilical cord pH values. For the mother, postpartum hemorrhage rates and puerperal hemoglobin status do not differ between early and delayed clamping groups (Andersson, 2013; Gomersall, 2021; Nudelman, 2020).
Of potential harms, a greater hemoglobin concentration raises risks for hyperbilirubinemia (Chap. 33, p. 606). Data are conflicting but suggest that neonatal phototherapy rates do not differ (Andersson, 2011; Chen, 2018; McDonald, 2013). That said, the American College of Obstetricians and Gynecologists (2020e) recommends that protocols should be in place that identify neonatal jaundice. Other cautions are cases requiring expedited maternal or fetal resuscitation. Others are those having abnormal placentation or compromised placental function, such as with abruption or fetal-growth restriction. Fewer data are available regarding cord “milking,” in which the operator pushes blood through the cord toward the newborn. This maneuver appears safe for term newborns and may be advantageous if rapid cord clamping is clinically indicated (Panburana, 2020).
For the preterm neonate, delayed cord clamping for 30 seconds or longer has several benefits. From systematic reviews on the topic, these include decreased rates of blood transfusion and death before hospital discharge (Fogarty, 2018; Rabe, 2019). However, one large randomized study did not show an advantage for its primary outcome, which was a composite of death plus major neonatal morbidity (arnow-Mordi, 2017). The American College of Obstetricians and Gynecologists (2020e) recommends delayed cord clamping for vigorous term and preterm neonates not needing immediate resuscitation at birth.
Cord milking in the preterm neonate may pose potential harm. Although conflicting, some data suggest higher associated rates of severe interventricular hemorrhage in those <32 weeks’ gestation (Katheria, 2019, 2020; Kumbhat, 2021). Because of rapid blood volume changes, organizations currently recommend against the routine use of cord milking for neonates born <29 weeks’ gestation (Seidler, 2021; Wycko, 2015).
■ Occiput Transverse Position
In some cases, pelvic shape leads to a persistent occiput transverse (OT) position that is not easily overcome during secondstage pushing. A platypelloid pelvis is flattened anteroposteriorly and an android pelvis is heart shaped (Fig. 2-16, p. 29). With these, space may be inadequate for occipital rotation to either an anterior or posterior position. In the absence of a pelvic architectural abnormality or asynclitism, the OT position is usually transitory. Thus, unless contractions are hypotonic, the head usually rotates spontaneously to an OA position. If rotation ceases because of poor expulsive forces, vaginal delivery can be aided. The easiest is manual rotation of the occiput either anteriorly to an OA or, less commonly, posteriorly to an OP position. Kielland forceps also can rotate the occiput anteriorly. Both manual and forceps rotations are described in Chapter 29 (p. 540).
■ Persistent Occiput Posterior Position
Approximately 2 to 10 percent of singleton, term, cephalic fetuses deliver in an OP position (Cheng, 2010). Many fetuses delivering OP are OA in early labor but malrotate during labor. Predisposing risks include epidural analgesia, nulliparity, greater fetal weight, and prior delivery with OP positioning (Cheng, 2006a; Gardberg, 2004; Lieberman, 2005). OT pelvic shapes, an anthropoid pelvis and narrow subpubic angle can predispose (Barth, 2015; Ghi, 2016).
Women with a persistent OP position have higher associated rates of prolonged second-stage labor, cesarean delivery, and operative vaginal delivery. For women who deliver vaginally, rates of blood loss and of OASIS are increased (Senécal, 2005).
Newborns delivered from an OP position have higher complication rates than those born positioned OA. In one study of neonatal outcomes in 2591 women undergoing delivery from a persistent OP position, rates of acidemic umbilical cord gases, birth trauma, Apgar scores <7, and intensive care nursery admission, among others, were higher compared with an OA position (Cheng, 2006b). Others report similar findings (Fitzpatrick, 2001; Ponkey, 2003).
Fetal head position assessment is essential to management (Chap. 22, p. 429). However, digital examination for identification of fetal head position can be inaccurate, and transabdominal sonography may increase accuracy (Bellussi, 2017). The transducer is placed transversely just cephalad to the maternal mons pubis. In the sonogram, fetal orbits and nasal bridge lie ventrally, whereas the occiput apposes the lower sacrum. Such information may explain prolonged second-stage labor and identify suitable candidates for rotation. Of other interventions, varying maternal position either antepartum or during labor does not appear to lower rates of persistent OP position (Kariminia, 2004; Le Ray, 2016). If the bony pelvic outlet is roomy and the perineum is somewhat relaxed from prior deliveries, rapid spontaneous OP delivery can take place. Conversely, if the perineum is resistant to stretch, second-stage labor may be appreciably prolonged. During each maternal push, the head is driven against the perineum to a greater degree than when the head position is OA. This leads to greater rates of OASIS (Groutz, 2011). For these reasons, manual rotation with spontaneous delivery from an OA position has advantages. Lower rates of cesarean delivery, severe perineal laceration, chorioamnionitis, and maternal blood loss follow rotation to OA position (Shaer, 2011). Alternatively, forceps rotation to an OA position may be attempted for those with suitable skills. Last, forceps or vacuum device also can be applied and delivery completed from an OP position. These operative vaginal techniques are detailed in Chapter 29 (p. 540).
Infrequently, protrusion of fetal scalp through the introitus can be erroneously encouraging. In these cases, findings reflect marked fetal head elongation from molding plus substantive scalp edema and not fetal descent. In some cases, the head may not even be engaged—that is, the biparietal diameter may not have passed through the pelvic inlet. Labor is characteristically long and descent of the head is slow. Careful palpation above the symphysis may disclose the fetal head to be above the pelvic inlet. Prompt cesarean delivery is appropriate.
SHOULDER DYSTOCIA
Following complete emergence of the fetal head during vaginal delivery, the remainder of the body may not rapidly follow. The anterior fetal shoulder can become wedged behind the symphysis pubis and fail to deliver with maternal pushing and gentle axial traction by the provider. One indicator may be retraction of the baby’s head against the mother’s perineum—the turtle sign. Because the umbilical cord is compressed within the birth canal, shoulder dystocia is an emergency. Several maneuvers may be performed to free the shoulder. This requires a team approach, and effective communication and leadership are critical.
Consensus regarding a specific definition of shoulder dystocia is lacking. Some, including the American College of Obstetricians and Gynecologists (2019c), diagnose it if maneuvers are required to free the shoulder. Spong and coworkers (1995) reported that the mean head-to-body delivery time in normal births was 24 seconds compared with 79 seconds in those with shoulder dystocia. These investigators proposed that a head-to-body delivery time >60 seconds be used to define shoulder dystocia. Currently, however, the diagnosis continues to rely on the clinical perception that the normal traction needed for fetal shoulder delivery is ineffective. Because of these differing definitions, the incidence of shoulder dystocia varies. One review cites a clinically useful average of 1 percent of all deliveries (Ouzounian, 2016). The incidence has risen in recent decades, likely due to increasing fetal birthweight (Øverland, 2014). Greater recognition and documentation also may boost reported incidences (Kim, 2016).
■ Maternal and Neonatal Consequences
In general, shoulder dystocia poses greater risk to the fetus than to the mother. The main maternal risks are serious perineal tears and postpartum hemorrhage, usually from uterine atony but also from lacerations (Hehir, 2020; Rahman, 2009). In contrast, significant neuromusculoskeletal injury and asphyxia are neonatal concerns. These specific injuries are described fully in Chapter 33 (p. 599). In one review of 1177 shoulder dystocia cases, brachial plexus injury was diagnosed in 11 percent and clavicular or humeral fracture in 2 percent (Chauhan, 2014). MacKenzie and associates (2007) reviewed 514 cases. Of the neonates, 7 percent showed evidence of acidosis at delivery, and 1.5 percent required cardiac resuscitation or developed hypoxic ischemic encephalopathy (HIE). In another review of 200 cases, rates of severe fetal acidosis and HIE were each 0.5 percent if delivery was completed within 5 minutes. These rates rose to 6 and 24 percent, respectively, with delivery delays ≥5 minutes (Leung, 2011).
■ Prediction and Prevention
Although several factors are clearly associated with this complication, shoulder dystocia cannot be accurately predicted or prevented (American College of Obstetricians and Gynecologists, 2019c). Associated characteristics include fetal macrosomia, maternal obesity, prolonged second-stage labor, operative vaginal delivery, and a prior shoulder dystocia (Mehta, 2004; Zhang, 2018).
Of these, increasing birthweight creates a corresponding rise in the incidence of shoulder dystocia (Øverland, 2012). With fetal macrosomia, commonly associated maternal characteristics are obesity, postterm pregnancy, multiparity, and diabetes (Koyanagi, 2013). The combination of fetal macrosomia and maternal diabetes mellitus escalates the frequency of shoulder dystocia (Nesbitt, 1998). This predisposition may stem from anthropomorphic differences between comparable-weight fetuses of nondiabetic and diabetic mothers. The latter fetal group has larger shoulder and extremity circum- ferences and greater shoulder-to-head and chest-to-head size (Modanlou, 1982). However, translating these specific measurements into stand-alone sonographic thresholds shows poor predictive sensitivity (Burkhardt, 2014). Planned cesarean delivery should be considered for the nondiabetic woman with a fetus whose estimated fetal weight is >5000 g or for the diabetic woman whose fetus is estimated to weigh >4500 g (American College of Obstetricians and Gynecologists, 2019c).
As prevention, early labor induction has been evaluated. In one study, approximately 800 women with suspected macrosomic fetuses were randomly assigned to early induction between 37 and 39 weeks’ gestation or to expectant care (Boulvain, 2015). Women with insulin-requiring diabetes were excluded. Dystocia rates were lowered by two thirds in the induction group, and neither group suffered brachial plexus injury. Other neonatal outcomes were similar, except for a higher rate of hyperbilirubinemia and phototherapy in the induction group. A systematic review of four randomized trials again with the same compared groups showed no differences in rates of cesarean delivery, shoulder dystocia, or operative vaginal delivery. Fetal fracture rates were lower in the induction group (MagroMalosso, 2017). Notably, when assessing the benefits, the poor accuracy of antepartum fetal weight prediction should be considered (Malin, 2016). Moreover, potential benefits of early induction are balanced against potential harms from early delivery. The American College of Obstetricians and Gynecologists (2019a,c) recommends against labor induction before 39 weeks for suspected macrosomia alone.
Of other risks, recurrent shoulder dystocia ranges from 4 to 13 percent in the three largest series (Al-Hawash, 2019). For many women with prior shoulder dystocia, labor may still be a reasonable option. The American College of Obstetricians and Gynecologists (2019c) recommends that estimated fetal weight, gestational age, maternal glucose intolerance, and severity of prior neonatal injury be evaluated. Antepartum, risks and benefits of cesarean delivery are discussed with any woman with a history of shoulder dystocia. After discussion and risk assessment, either mode of delivery is appropriate.
FIGURE 27-2 The McRoberts maneuver. The maneuver consists of removing the legs from the stirrups and sharply flexing the thighs up onto the abdomen. Simultaneously, an assistant also provides suprapubic pressure that is directed down and lateral■ Management
Shoulder dystocia cannot be accurately predicted. Tus, the labor and delivery team, which includes nurses, obstetrical providers, and anesthesia sta, should be well versed in its management. An emergent call or assistance should assemble these members. Because o ongoing cord compression with this dystocia, one goal is to reduce delivery time. Tis is balanced against the second goal, which is avoiding etal and maternal injury rom aggressive manipulations. Adequate analgesia assists maneuvers. Episiotomy may be needed to provide sucient room or essential manipulations but itsel does not lower brachial plexus injury rates (Gurewitsch, 2004; Paris, 2011).
Various techniques can be used to ree the anterior shoulder rom its impacted position behind the symphysis pubis. Te McRoberts maneuver is oten selected rst. With it, legs are lited rom stirrups, hips are sharply exed up onto the abdomen, and knees remain exed. Gherman and colleagues (2000) analyzed the McRoberts maneuver using x-ray pelvimetry. Te procedure causes straightening o the sacrum relative to the lumbar vertebrae, rotation o the symphysis pubis toward the maternal head, and a decrease in the angle o pelvic inclination.
Tis does not increase pelvic dimensions, but pelvic rotation cephalad tends to ree the impacted anterior shoulder. Gonik and coworkers (1989) tested the McRoberts position objectively with laboratory models and ound that the maneuver reduced the orces needed to ree the etal shoulder. During McRoberts maneuver, an assistant can apply downward and laterally directed suprapubic pressure (Fig. 27-2). Tis abducts the anterior shoulder to create a shorter bisacromial diameter.
It also rotates the shoulders into the oblique diameter, which at the pelvic inlet is longer than the anteroposterior diameter. I unsuccessul, most move next to the posterior shoulder or to rotation maneuvers.
FIGURE 27-3 Delivery of the posterior shoulder for relief of shoulder dystocia. A. The operator’s hand is introduced into the vagina along the fetal posterior humerus. B. The arm is splinted and sweptacross the chest, keeping the arm flexed at the elbow. C. The fetal hand is grasped and the arm extended along the side of the face. The posterior arm is delivered from the vagina.
With delivery o the posterior shoulder, the provider inserts a hand into the posterior pelvis, careully sweeps the posterior arm o the etus orward across its chest, and delivers the arm (Fig. 27-3). I possible, the operator’s ngers are aligned parallel to the long axis o the etal humerus to lower racture risks. With the arm now outside o the pelvis, the bisacromial diameter is diminished. Next, the shoulder girdle is rotated into one o the oblique diameters o the pelvic inlet. Tese steps resolve the disproportion to ree the anterior shoulder. O the rotational maneuvers, the method recommended by Rubin (1964) aims to shorten the bisacromial diameter (Fig. 27-4). A pelvic hand reaches the most easily accessible etal shoulder, oten the posterior one, which is then pushed toward the anterior surace o the chest. Tis maneuver abducts the posterior shoulder and rotates both shoulders into one o the pelvic inlet’s oblique diameters. Friction rom the vagina also abducts the anterior shoulder to some degree during this rotation. Again, resolution o the disproportion can ree the anterior shoulder.
FIGURE 27-4 Rubin maneuver. The bisacromial diameter is aligned vertically. The more easily accessible fetal shoulder (the anterior is shown here) is pushed toward the anterior chest wall of the fetus (arrow). Most often, this results in abduction of both shoulders, which reduces the bisacromial diameter and frees the impacted anterior shoulder.Instead, Woods (1943) reported that by rotating the posterior shoulder progressively in a corkscrew ashion, the impacted anterior shoulder could be released. For perormance, one hand is pressed against the anterior surace o the posterior shoulder, and pressure is directed toward the etal back. Te posterior shoulder is then rotated 180 degrees, and in doing so, it becomes the anterior-positioned limb. With rotation, this shoulder comes to lie beneath, not behind, the symphysis and can be delivered.
In a review o these our methods, all had similar neonatal injury rates (Spain, 2015). Importantly, delivery duration and the number o maneuvers raises neonatal injury rates. I the above are initially unsuccessul, they may be repeated, and other methods may be elected. With an all-ours maneuver, also called the Gaskin maneuver, the parturient rolls onto her hands and knees. Here, axial traction against the head and neck attempts to ree the posterior shoulder (Bruner, 1998). Challenges with this include immobility rom regional analgesia and time lost in patient repositioning.
In some cases, the posterior arm is inaccessible or delivery. Cluver and Homeyr (2009) described posterior axilla sling traction to deliver the posterior arm. With this, suction tubing or a sti urinary catheter is threaded under the etal axilla, and both ends are brought together above the shoulder. Tis orms a loop beneath the axilla. Catheter traction then sweeps upward and outward to deliver the shoulder. Data continue to accrue. In a case series o 119 cases in which this was the rst maneuver, the success rate was 96 percent (Ansell, 2019).
For reractory cases despite the above eorts, deliberate racture o the anterior clavicle by using the thumb to press it toward and against the maternal pubic ramus can be attempted to ree the shoulder impaction. In practice, however, deliberate racture o a large neonate’s clavicle is dicult. I successul, the racture will heal rapidly and is usually trivial compared with brachial nerve injury, asphyxia, or death. Symphysiotomy involves cutting o the intervening symphyseal cartilage and much o its ligamentous support to widen the symphysis pubis. In rare cases, it has been used successully or shoulder dystocia (Goodwin, 1997). Using local analgesia, symphysiotomy surgically divides the intervening symphyseal cartilage and much o its ligamentous support to widen the symphysis pubis up to 2.5 cm (Basak, 2011). I easible, a previously inserted urinary catheter is displaced rom the midline with the index nger o the let hand, which is inside the vagina. Lack o provider training and potentially serious maternal pelvic or urinary tract injury explain its rare use in the United States.
Te Zavanelli maneuver involves replacement o the etal head into the pelvis ollowed by cesarean delivery (Sandberg, 1985). erbutaline, 0.25 mg, is given subcutaneously to produce uterine relaxation. Te operator rst turns the head to an OA or OP position, exes it, and slowly elevates it back into the vagina. Cesarean delivery is then perormed. Sandberg (1999) reviewed 103 reported cases. It was successul in 91 percent o cephalic cases and in all cases o breech head entrapments. Despite successul replacement, etal injuries were common but may have resulted rom the multiple manipulations used beore the Zavanelli maneuver (Sandberg, 2007). Cleidotomy consists o cutting the clavicle with scissors or other sharp instruments. It is usually done or a dead etus (Schramm, 1983).
In preparation or shoulder dystocia, clinical simulations aim to improve perormance, documentation, and retention o drill steps. Teir use has translated into improved neonatal outcome in some, but not all, investigations (Crots, 2016; Fransen, 2017; Gurewitsch Allen, 2017; Walsh, 2011). At our institution, these drills involve resident, aculty, nursing, and anesthesia sta.
SPECIAL POPULATIONS
■ Home Birth
In 2017, 1.4 percent o deliveries in the United States were planned home births. Unplanned home births constituted 0.2 percent (MacDorman, 2019). O unplanned home births in a 15-year epoch in Norway, 69 o 6027 or 1.1 percent resulted in etal or neonatal death. Tis high rate was attributable to inection, prematurity, and placental abruption (Gunnarsson, 2017). Multiparity and distance rom the hospital were ascribed risks (Gunnarsson, 2014). In the United States, youth, lack o prenatal care, minority race, and lower educational attainment are risks (Declercq, 2010).
With planned home birth o a low-risk pregnancy, benets include ewer medical interventions that include labor augmentation, episiotomy, operative vaginal delivery, and cesarean delivery (Bolten, 2016; Cheyney, 2014). Regarding the saety o planned home birth, data rom randomized trials are lacking, and large observational studies derive rom heterogeneous care systems, whose results may not be generalizable. For example, several developed countries deliver a large volume o careully screened women at home. Gravidas are attended by midwives with substantial training and in a setting closely integrated with the local health-care system (Comeau, 2018; de Jonge, 2015). Te level o such coordination in the United States is less uniorm.
Overall, neonatal risks o home births in the United States are small but greater than those o hospital delivery. Midwieattended home births carry a neonatal mortality risk o 1.3 per 1000 births. Tis is a nearly ourold greater rate compared with midwie-attended hospital births (Grünebaum, 2020). Te most common underlying causes o death are those attributed to labor and delivery events, to congenital anomalies, and to inection. O neonatal injuries, rates o neonatal seizure and serious neurological dysunction also are elevated in home-birth groups (Grünebaum, 2013, 2014, 2017; Snowden, 2015). Importantly, substantial risks attend home birth or those with preeclampsia, with multietal gestation, with prior cesarean delivery, or with breech presentation (Caughey, 2019). Te American College o Obstetricians and Gynecologists (2020b) specically considers the last three to be absolute contraindications. Further, the College considers accredited hospitals and birthing centers to be the saest site or birth but recognizes the autonomy o the well-counseled patient.
■ Water Birth
As one option or pain relie, some women choose to spend part o rst-stage labor in a large water tub. With this practice, one Cochrane review ound lower rates o regional analgesia use and no greater adverse neonatal or maternal eects compared with traditional labor (Cluett, 2018).
For second-stage labor, water birth similarly aims to relieve pain, but it can pose neonatal risks. Te rate o cord avulsion during water birth approximates 3 per 1000 births and stems primary rom abruptly bringing the newborn out o the water (Schaer, 2014). Fresh-water drowning and pneumonia with sepsis are reported causes o neonatal death (Byard, 2010). Te latter emphasizes the need or rigorous sanitizing protocols. Systematic reviews comment on the paucity o high-quality data but do not identiy overall greater neonatal or maternal harms or benets rom water birth in low-risk, term, singleton gestations (Cluett, 2018; aylor, 2016). Given the lack o robust data and potential or serious complications, the American College o Obstetricians and Gynecologists (2016) currently recommends that “birth occur on land, not in water.”
■ Female Genital Mutilation
This practice refers to medically unnecessary vulvar and perineal surgical modification. In the United States, it is a federal crime to perform unnecessary genital surgery on a girl younger than 18 years and is condemned by major health organizations that include the American College of Obstetricians and Gynecologists (2019b). Despite this, forms of female genital mutilation are practiced in countries throughout Africa, the Middle East, and Asia (UNICEF, 2020).
The World Health Organization (WHO) (2018a) classifies female genital mutilation (FGM) into four types (Table 27-1). Some long-term risks include propensity for urogenital infection, chronic vulvar pain, and dyspareunia (Berg, 2014b). Thus, early in care, new gravidas are asked about these problems or prior obstetrical complications. The psychiatric consequences also can be profound, and referral for psychological assessment can be offered (Low-Beer, 2018 With physical examination, a finger or cotton swab assesses elasticity and caliber of the vaginal opening. Parturients with types I, II, and IV typically require only routine obstetrical care. Those with type III FGM or with extensive scarring have higher delivery risks. If an obstruction to childbirth is recognized, the advantages of surgical release are ideally discussed early antenatally. Division of midline scar tissue to reopen the vulva is termed defibulation but also called deinfibulation or anterior episiotomy (Kalis, 2012).
FGM is associated with some adverse maternal and neonatal complications. Moreover, vaginal obstruction can delay diagnosis of other pregnancy conditions. From a prospective study, the WHO (2006) estimated that FGM raised perinatal morbidity rates by 1 to 2 percent. For mothers with FGM, rates for prolonged labor, perineal lacerations and episiotomy, and postpartum hemorrhage were higher than those without prior FGM (Berg, 2014a; Chibber, 2011). Evidence supports that gravidas with type III FGM who undergo defibulation benefit from lower rates of cesarean delivery and OASIS (Berg, 2018; Rodriguez, 2016). To prevent obstetrical complications, the WHO (2016) recommends defibulation, which can be completed antepartum or intrapartum (Fig. 27-5). In our and other’s experiences, intrapartum defibulation allows successful vaginal delivery without major complications (Rouzi, 2012).
In discussing defibulation, cultural sensitivity is essential, and some women may be offended by the suggestion that they have been assaulted or mutilated (American College of Obstetricians and Gynecologists, 2014). Preoperative counseling ideally discusses expected anatomic and physiologic changes. Partial defibulation uncovers the vagina and urethra, and total defibulation extends the incision to reveal the clitoris, if present. Women with infibulation often have a slow urine stream, and they perceive menses and urine to originate from the same opening. Both will change after scar incision. Affected women may be unfamiliar with normal vaginal anatomy, which will be visible following surgery. One excellent review is provided by Abdulcadir and associates (2018). Wound complications such as hematoma, cellulitis, or incisional dehiscence are rare but are discussed during counseling. Postoperative dyspareunia is uncommon, and in most studies, female sexual functioning scores improve (Berg, 2018; Nour, 2006).
FIGURE 27-5 Deinfibulation. Although not shown here, lidocaine is first infiltrated along the planned incision if regional analgesia is not in place already. To protect underlying structures, two fingers or the tips of a narrow clamp are insinuated behind the shelf created by fused labia but in front of the urethra and crowning head. The shelf is then incised in the midline. After delivery, the raw edges are sutured with rapidly absorbable material to secure hemostasis. (Reproduced with permission from Hawkins JS: Lower genital tract procedures. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)■ Prior Pelvic Reconstructive Surgery
These surgeries are performed with increasing frequency in reproductive-aged women, and thus pregnancy following them is not uncommon. Major concerns are the degradation of repair by pregnancy and by vaginal birth. After midurethral sling surgery and then a subsequent pregnancy, continence is preserved for most. Multiple pregnancies, however, may raise recurrence risk. Evidence does not favor a delivery route, and vaginal delivery is suitable (Adams-Piper, 2016; Cavkaytar, 2014; Dyrkorn, 2020). Sacral neuromodulation units are recommended to be turned of because of unknown pregnancy effects from chronic electrical stimulation. From small reviews, lowered device efficacy and displaced or broken device leads can follow both vaginal and cesarean delivery (Mahran, 2017; Roulette, 2018).
Data do not proscribe vaginal birth.
Hysteropexy aims to correct prolapse of the vaginal apex yet preserve the uterus. Available case series are few and small and do not allow estimates of recurrence rate or delivery route recommendations (Wieslander, 2020). Most undergo elective cesarean delivery.
■ Anomalous Fetuses
Rarely, delivery can be obstructed by extreme hydrocephaly, body stalk anomaly, conjoined twins, or massive fetal abdominal enlargement from a greatly distended bladder, ascites, or organomegaly. Specifically, with milder forms of hydrocephaly, if the biparietal diameter is <10 cm or if the head circumference is <36 cm, vaginal delivery may be permitted (Anteby, 2003). In rare cases in which neonatal death has occurred or is certain due to associated anomalies, vaginal delivery may be reasonable, but the head or abdomen must be reduced in size for delivery. Removal of fluid by cephalocentesis or paracentesis with sonographic guidance can be performed intrapartum. As described earlier, cleidotomy can shorten the bisacromial diameter. For hydrocephalic fetuses that are breech, cephalocentesis can be accomplished suprapubically when the aftercoming head enters the pelvis. Currently, these practices are more germane in developing countries.
THIRD STAGE OF LABOR
■ Delivery of the Placenta
Third-stage labor begins immediately after fetal birth and ends with placental delivery. Goals include delivering an intact placenta and avoiding uterine inversion or postpartum hemorrhage. The latter two, described in Chapter 42 (p. 731), are intrapartum emergencies. Immediately after newborn birth, uterine fundal size and consistency are examined. If the uterus remains firm and bleeding is minimal, watchful waiting until the placenta separates is the usual practice. Neither massage nor downward fundal pressure is employed, but the fundus is frequently palpated to ensure that it does not become atonic and filled with blood from placental separation. To prevent uterine inversion, umbilical cord traction must not be used to pull the placenta from the uterus. Signs of separation include a sudden gush of blood into the vagina, a globular and firmer fundus, outward movement of the umbilical cord as the placenta descends into the vagina, and elevation of the uterus into the abdomen. With the last, the placenta, having separated, passes down into the vagina. Here, its bulk pushes the uterine body upward.
These signs appear within minutes after newborn delivery, and the median time ranges from 4 to 12 minutes (Frolova, 2016; Shinar, 2016b). Once the placenta has detached from the uterine wall, the mother can bear down, and intraabdominal pressure often expels the placenta into the vagina. These efforts may fail or may not be possible because of analgesia. After ensuring that the uterus is contracted firmly, the umbilical cord is kept slightly taut but is not pulled. Pressure is exerted by a hand wrapped around the fundus to propel the detached placenta into the vagina (Fig. 27-6). Concurrently, the heel of the same hand exerts pressure between the symphysis pubis and the uterine fundus and directs it toward the sacrum. This aims to prevent inversion. Once the placenta passes through the introitus, pressure on the uterus is relieved. The placenta is then gently lifted away. Care is taken to prevent placental membranes from being torn of and left behind. If the membranes begin to tear, they are grasped with a clamp and removed by gentle teasing (Fig. 27-7).
FIGURE 27-6 Expression of the detached placenta. Note that the hand is not trying to push the fundus through the birth canal! As the placenta leaves the uterus and enters the vagina, the uterus is pushed cephalad by the heel of the hand on the abdomen while the cord is held in position. The mother can aid in the delivery of the placenta by bearing down. As the placenta reaches the perineum, the cord is lifted, which in turn lifts the placenta out of the vagina.FIGURE 27-7 Membranes that were somewhat adhered to the uterine lining are separated by gentle traction with ring forceps.
■ Management of the Third Stage
Practices within this stage of labor may be broadly considered as either expectant or active. Expectant management involves waiting for placental separation signs and allowing the placenta to deliver either spontaneously or aided by nipple stimulation or gravity (World Health Organization, 2012). In contrast, active management of third-stage labor includes earlier cord clamping, controlled cord traction during placental delivery, and immediate prophylactic administration of a uterotonic agent. The goal of this triad is to limit postpartum hemorrhage. However, the value of this bundled approach is questioned (Begley, 2019). For example and as noted previously (p. 500), delayed cord clamping does not raise postpartum hemorrhage rates, and thus early clamping is a less important component of this trio. Similarly, cord traction fails to prevent hemorrhage. fraction does lower manual placenta removal rates and shortens third-stage labor (Deneux-Taraux, 2013; Homeyr, 2015). Following placental delivery, we support uterine massage to prompt uterine firming but recognize that evidence for its benefits to avoid postpartum hemorrhage is not strong (Saccone, 2018).
Therefore, uterotonic drugs play an essential preemptive role to decrease postpartum blood loss (Salati, 2019). A single agent is given either before or after placental expulsion, and timing does not affect rates of postpartum hemorrhage, placental retention, or third-stage labor length (Soltani, 2010). Choices for hemorrhage prophylaxis include oxytocin (Pitocin), misoprostol (Cytotec), and the ergots, namely ergonovine (Ergotrate) and methylergonovine (Methergine). In addition, a combination agent of oxytocin plus ergonovine (Syntometrine) is used outside the United States. Also in other countries, carbetocin (Duratocin), a long-acting oxytocin analogue, is available and effective for hemorrhage prevention (Kalaat, 2021). Prescribing information for carbetocin cites its use for cesarean delivery, but randomized trials reflect similar use for vaginal birth. Of agents, the WHO (2018) recommends oxytocin for first-line use.
High-dose Oxytocin
Synthetic oxytocin is identical to that produced by the posterior pituitary. Its action is noted at approximately 1 minute, and it has a mean half-line of 3 to 5 minutes. Recommended storage temperature is ≤25C°, which may pose problems in some lowresource countries. When given as a bolus, oxytocin can cause profound hypotension (Svanström, 2008). This hemodynamic change may be dangerous to women with hemorrhage-related hypovolemia or with certain cardiac conditions. Thus, oxytocin should be given as a dilute solution by continuous intravenous infusion or as an intramuscular injection.
Despite the routine use of oxytocin, no standard prophylactic dose has been established for its use following either vaginal or cesarean delivery. Water intoxication can result from the antidiuretic action of high-dose oxytocin if administered in a large volume of electrolyte-free dextrose solution (Whalley, 1963). Our practice is to add 20 units (2 mL) of oxytocin per liter of crystalloid solution. This is administered after delivery of the placenta at a rate of 10 to 20 mL/min—200 to 400 mU/min—for a few minutes until the uterus remains firmly contracted and bleeding is controlled. The infusion rate then is reduced to 1 to 2 mL/min until the mother is ready for transfer to the postpartum unit. The infusion is usually then discontinued. For women without intravenous access, 10 units of intramuscular oxytocin are injected.
Other Agents
Ergonovine and methylergonovine have similar activity in myometrium, but only methylergonovine is currently manufactured in the United States. These ergot alkaloid agents lower the rates of postpartum hemorrhage and the need for additional uterotonic drugs. However, blood pressure elevation is a concerning side effect (Liabsuetrakul, 2018). Methylergonovine is contraindicated in hypertensive women. If selected, a 200-μg dose of methylergonovine is given intramuscularly or is slowly injected intravenously in a period not less than 60 seconds to avoid sudden hypertension.
Misoprostol is a prostaglandin E1 analogue, which has proved inferior to oxytocin for postpartum hemorrhage prevention (unçalp, 2012). However, in resource-poor settings that lack oxytocin, misoprostol is suitable for hemorrhage prophylaxis and is given orally as a single 400- or 600-μg dose (World Health Organization, 2018c). Side effects include fever and diarrhea. From systematic reviews, carbetocin and oxytocin show equivalent efficacy for postpartum hemorrhage prevention after vaginal birth (van der Nelson, 2021; Widmer, 2018). Carbetocin is more expensive. A heat-stable form may have benefit in low-resource settings that may have inconsistent temperatures during supply distribution (Malm, 2018).
Tranexamic acid (TXA) is an antifibrinolytic agent and has been evaluated to prevent postpartum hemorrhage (Ahmadzia, 2018). In one metaanalysis, a 1-g intravenous TXA dose following delivery in addition to oxytocin was reported to lower the hemorrhage rate from 11.4 to 8.7 percent (Saccone, 2020). In a randomized trial of 3891 women, however, TXA plus oxytocin did not decrease the postpartum hemorrhage incidence compared with oxytocin alone (Sentilhes, 2018). Currently, TXA use is not recommended prophylactically (American College of Obstetricians and Gynecologists, 2019c). To treat hemorrhage, TXA use is described in Chapters 42 (p. 736) and 44 (p. 773).
■ Manual Removal of Placenta
In approximately 2 percent of singleton births, the placenta may not deliver promptly (Cheung, 2011). This may represent: (1) placenta adherens, in which uterine contractions are insufficient to detach the placenta; (2) lower uterine segment constriction and a detached but trapped placenta; or (3) placenta accreta spectrum. Consistent risks for retained placenta include stillbirth, prior cesarean delivery, prior retention, and preterm delivery (Belachew, 2014; Coviello, 2015; Endler, 2014). For the last, in one study with nearly 46,000 deliveries, analysis predicted that 90 percent of placentas would spontaneously deliver by 180 minutes for gestations at 20 weeks; 21 minutes at 30 weeks; and 14 minutes at 40 weeks (Dombrowski, 1995).
Postpartum hemorrhage can complicate a retained placenta, and bleeding risk accrues with the length of third-stage labor. In the absence of bleeding, evidence supports 15 to 20 minutes (Frolova, 2016; Shinar, 2016a; van Ast, 2019). The WHO (2012) cites a 60-minute threshold. Notably, if brisk bleeding ensues and the placenta cannot be delivered by standard technique, manual removal of the placenta is indicated (Fig. 27-8). When performed, some administer a single dose of intravenous antibiotics, however, one systematic review of observational studies found no benefits (Chibueze, 2015). The American College of Obstetricians and Gynecologists (2020d) concluded that data neither support nor reute this practice, but the WHO (2012) recommends prophylaxis. At our institution, we administer a single dose of cefazolin to women not already receiving antibiotics and not penicillin allergic.
IMMEDIATE POSTPARTUM CARE
FIGURE 27-8 Manual removal of placenta. A. One hand grasps the fundus and the other hand is inserted into the uterine cavity and the fingers are swept from side to side as they are advanced. B. When the placenta detaches, it is grasped and removed.The hour immediately following delivery of the placenta is critical. During this time, lacerations are repaired. Although uterotonic agents are administered, postpartum hemorrhage as the result of uterine atony is most likely at this time. Hematomas may expand. Consequently, uterine tone and the perineum are frequently evaluated. The mother’s blood pressure and pulse are recorded immediately after delivery and every 15 minutes for the first 2 hours. Her temperature is recorded every 4 hours during the first 8 hours and then every 8 hours thereafter. Vital signs may be obtained more frequently in patients at higher risk of postpartum complications (American Academy of Pediatrics, 2017). The placenta, membranes, and umbilical cord are examined for completeness and anomalies, as described in Chapter 6 (p. 107).
■ Skintoskin Contact
This practice aims to connect the mother and newborn dyad early to promote breastfeeding (American College of Obstetricians and Gynecologists, 2020a; Moore, 2016; World Health Organization, 2018b). Ideally, the mother lies in a semireclined position. After the newborn is dried to prevent cooling, its chest is placed against the mother’s breast with access to her nipple. Its legs are flexed, and the mother’s arm steadies its body. Covered with warm blankets and often a cap, the neonate’s head is uncovered, turned to the side, and placed in a sniffing position. Mouth and nares should remain unobstructed (Widström, 2019). The dyad is continually monitored for physiologic stability, somnolence, unobstructed breathing, and safe positioning to avoid smothering or falls (FeldmanWinter, 2016).
■ Lower Genital Tract Lacerations
These lacerations may involve the cervix, vagina, or perineum. Those of the cervix and vagina are described in Chapter 42 (p. 739). In general, those of the perineum follow vaginal delivery, and most are first- and second-degree lacerations. Lacerations are classified by depth and involved anatomy, and complete definitions and visual examples are given in Figure 27-9. Of these, third-degree lacerations involve the anal sphincter and are subcategorized as:
(3a) <50 percent external anal sphincter (EAS) tear;
(3b) >50 percent EAS tear; and
(3c) EAS plus internal anal sphincter (IAS) tears.
Third- and fourth-degree perineal lacerations are considered obstetric anal sphincter injuries (OASIS), and their combined incidence is 0.1 to 5 percent (Blondel, 2016; Friedman, 2015). Risk factors for these more complex lacerations include nulliparity, midline episiotomy, persistent OP position, Asian race, increasing fetal birthweight, and operative vaginal delivery (Gurol-Urganci, 2013; Landy, 2011). Morbidity rates rise as laceration severity increases. Compared with simpler lacerations, OASIS are associated with greater blood loss and puerperal pain. Wound disruption and infection rates are other risks (Goldaber, 1993; LewickyGaupp, 2015). Stock and coworkers (2013) reported that approximately 7 percent of 909 OASIS had complications. Long term, OASIS are linked with approximately doubled rates of anal incontinence compared with vaginal delivery without OASIS (Evers, 2012; Schei, 2019). Fourth-degree lacerations pose greater anal incontinence risk than third-degree ones (Gommesen, 2020; Jangö, 2018). Evidence implicating OASIS in subsequent long-term dyspareunia are conflicting (Mous, 2008; O’Shea, 2018). To ensure appropriate repair, identification and correct categorization is essential. Intrapartum endoanal ultrasound has been used to unmask clinically occult tears (Faltin, 2005).
Few data currently support this imaging intrapartum, and the American College of Obstetricians and Gynecologists (2020c) does not recommended it. For women with a prior OASIS, questions may arise as to subsequent pregnancy. In those without anal incontinence after OASIS repair, the rate of developing this incontinence is not increased by a subsequent vaginal birth (Jangö, 2016; Webb, 2017). Thus, a vaginal route is reasonable. However, women with a prior OASIS do have a higher recurrence rate with a subsequent delivery compared with multiparas without a prior OASIS (Edozien, 2014; Elfaghi, 2004). The risk mirrors that of primiparas in the general population and is low (Basham, 2013; Boggs, 2014). But, in those who experience a recurrent OASIS, the risk of anal incontinence can be substantial (Jangö, 2017). Fetal macrosomia and operative vaginal delivery are factors for OASIS recurrence and can influence counseling in future pregnancies. Specifically, patients may choose cesarean delivery to avoid repeat OASIS. This consideration may be most pertinent for those with prior postpartum anal incontinence, with OASIS complications that required a second repair, or with psychological trauma (American College of Obstetricians and Gynecologists, 2020c). Planned cesarean delivery is balanced against its associated operative risks (Chap. 30, p. 548).
FIGURE 27-9 1. First-degree perineal laceration: injury to only the vaginal epithelium or perineal skin. 2. Second-degree laceration: injury to perineum that spares the anal sphincter complex but involves the perineal muscles, which are the bulbospongiosus and superficial transverse perineal muscles. 3a. Third-degree laceration: <50 percent of the external anal sphincter (EAS) is torn. 3b. Third-degree laceration: >50 percent of the EAS is torn, but the internal anal sphincter (IAS) remains intact. 3c. Third-degree laceration: EAS and IAS are torn. 4. Fourth-degree laceration: the perineal body, entire anal sphincter complex, and anorectal mucosa are lacerated. (Reproduced with permission from Kenton K, Mueller M: Episiotomy and obstetric anal sphincter lacerations. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)■ Episiotomy
FIGURE 27-10 A mediolateral episiotomy is cut as the baby’s head crowns. Fingers are insinuated between the perineum and head. The incision begins in the midline and is directed toward the ipsilateral ischial tuberosity at an angle 60 degrees off the midline. (Reproduced with permission from Kenton K, Mueller M: Episiotomy and obstetric anal sphincter lacerations. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)FIGURE 27-11 Midline episiotomy repair. A. An anchor stitch is placed above the wound apex to begin a running, locking closure with 2–0 suture to close the vaginal epithelium and deeper tissues and reapproximate the hymeneal ring. B. A transition stitch redirects suturing from the vagina to the perineum. C. The superficial transverse perineal and bulbospongiosus muscles are reapproximated using a continuous, nonlocking technique with the same length of suture. This aids restoration of the perineal body for long-term support. D. The continuous suture is then carried upward as a subcuticular stitch. The final knot is tied proximal to the hymeneal ring. (Reproduced with permission from Kenton K, Mueller M: Episiotomy and obstetric anal sphincter lacerations. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
FIGURE 27-12 In overview, with end-to-end approximation of the external anal sphincter (EAS), a suture is placed through the EAS muscle, and four to six simple interrupted 2–0 or 3–0 sutures of polyglactin 910 are placed at the 3, 6, 9, and 12 o’clock positions through the perisphincter connective tissue. To begin, disrupted ends of the striated EAS muscle and capsule are identified and grasped. The first suture is placed posteriorly to maintain clear exposure. Another suture is then placed inferiorly at the 6 o’clock position. The sphincter muscle fibers are next reapposed by a figure-of-eight stitch. Last, the remainder of the fascia is closed with a stitch placed anterior to the sphincter cylinder and again with one placed superior to it. (Reproduced with permission from Kenton K, Mueller M: Episiotomy and obstetric anal sphincter lacerations. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
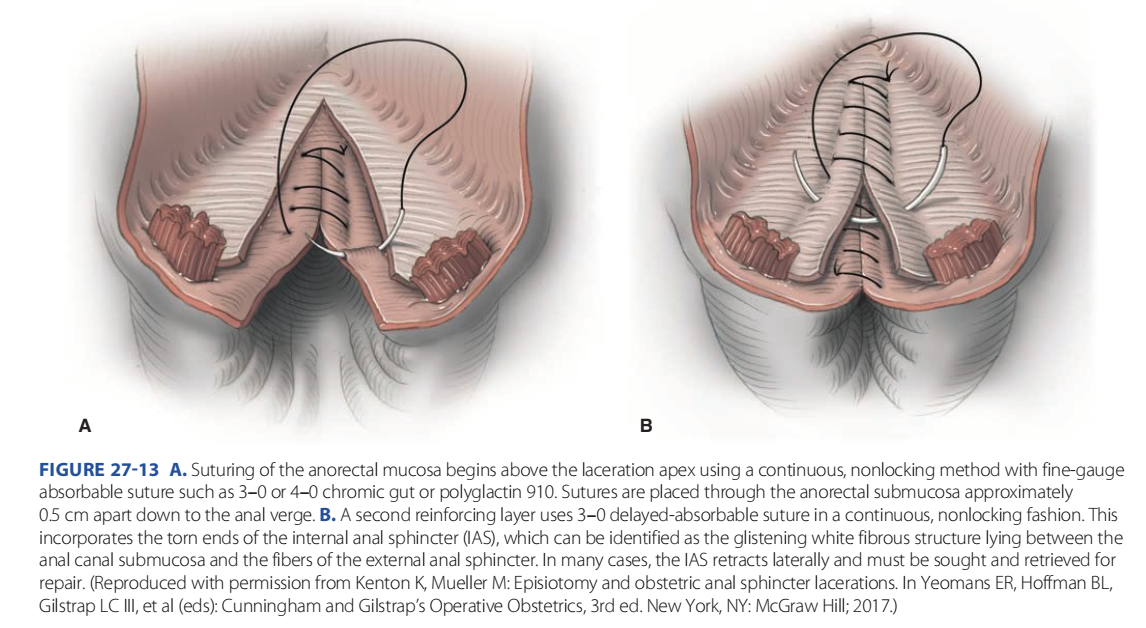
FIGURE 27-13 A. Suturing of the anorectal mucosa begins above the laceration apex using a continuous, nonlocking method with fine-gauge absorbable suture such as 3–0 or 4–0 chromic gut or polyglactin 910. Sutures are placed through the anorectal submucosa approximately 0.5 cm apart down to the anal verge. B. A second reinforcing layer uses 3–0 delayed-absorbable suture in a continuous, nonlocking fashion. This incorporates the torn ends of the internal anal sphincter (IAS), which can be identified as the glistening white fibrous structure lying between the anal canal submucosa and the fibers of the external anal sphincter. In many cases, the IAS retracts laterally and must be sought and retrieved for repair. (Reproduced with permission from Kenton K, Mueller M: Episiotomy and obstetric anal sphincter lacerations. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al (eds): Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
In contrast to spontaneous perineal tears, episiotomy is intended incision of the perineum. Its goal is enlargement of vaginal opening for birth. Textbooks and organizational guidelines often differ in their description. Kalis and colleagues (2012) presents one classification, and we agree with the need for terminology standardization. Midline and mediolateral episiotomies are the two main types and vary by the angle of perineal incision. Involved structures mirror those found with second-degree laceration, and their repairs are analogous. The midline episiotomy begins at the fourchette, incises the perineal body in the midline, and ends well before the EAS is reached. The chosen incision length varies from 2 to 3 cm depending on perineal length, degree of tissue thinning, and outlet diameter needs for delivery.
The mediolateral episiotomy begins at the midline of the fourchette Webb, 2017). Thus, a vaginal route is reasonable. However, women with a prior OASIS do have a higher recurrence rate with a subsequent delivery compared with multiparas without a prior OASIS (Edozien, 2014; Elfaghi, 2004). The risk mirrors that of primiparas in the general population and is low (Basham, 2013; Boggs, 2014). But, in those who experience a recurrent OASIS, the risk of anal incontinence can be substantial (Jangö, 2017). Fetal macrosomia and operative vaginal delivery are factors for OASIS recurrence and can influence counseling in future pregnancies. Specifically, patients may choose cesarean delivery to avoid repeat OASIS. This consideration may be most pertinent for those with prior postpartum anal incontinence, with OASIS complications that required a second repair, or with psychological trauma (American College of Obstetricians and Gynecologists, 2020c). Planned cesarean delivery is balanced against its associated operative risks (Chap. 30, p. 548).
■ Episiotomy
In contrast to spontaneous perineal tears, episiotomy is intended incision of the perineum. Its goal is enlargement of vaginal opening for birth. Textbooks and organizational guidelines often differ in their description. Kalis and colleagues (2012) presents one classification, and we agree with the need for terminology standardization. Midline and mediolateral episiotomies are the two main types and vary by the angle of perineal incision. Involved structures mirror those found with second-degree laceration, and their repairs are analogous. The midline episiotomy begins at the fourchette, incises the perineal body in the midline, and ends well before the EAS is reached. The chosen incision length varies from 2 to 3 cm depending on perineal length, degree of tissue thinning, and outlet diameter needs for delivery. The mediolateral episiotomy begins at the midline of the fourchette comparable (Kettle, 2010). However, traditional Vicryl occasionally requires removal of residual suture from the repair site because of pain. Ths disadvantage may be reduced by selecting a rapidly absorbed polyglactin 910 suture (Vicryl Rapide) (Greenberg, 2004; Kettle, 2002). Other delayed absorbable options include Monocryl or polydioxanone (PDS II). The perineal skin is closed by a running subcuticular stitch with the same suture type used to close the perineal defect.
For third-degree laceration repair, two methods are available to repair the EAS. The first is an end-to-end technique, which we prefer, and is shown in Figure 27-12. Initially, the torn ends of the EAS, which often retract, are isolated. The EAS and its surrounding connective tissue are brought to the midline. As a misnomer, this connective tissue is often called a capsule (Maldonado, 2020). The strength of this closure is derived from the connective tissue surrounding the sphincter and less so from the striated muscle. Thus, serial interrupted sutures incorporate sphincter fibers and a substantial portion of the perisphincter connective tissue to bring EAS ends together. If the IAS is torn, a running, nonlocking closure is completed with 3-0 or 4-0 suture (see Fig. 27-13B). Few data guide suture selection for sphincter repair, but delayed-absorbable material can provide sustained tensile strength during healing (Williams, 2006). This theory is supported by the study by Jallad and coworkers (2016), which showed a higher perineal breakdown rate following OASIS repair with chromic catgut. With the overlapping technique, the ends of the EAS are brought to the midline, lie atop one another, and are sutured in this position. This method is suitable only for type 3c lacerations—those with complete EAS rupture. Two rows of mattress sutures travel through both overlapping EAS ends to recreate the anal ring. In comparing the two methods, neither yields superior long-term anatomical or functional results (Farrell, 2012; Fernando, 2013; Fitzpatrick, 2000). Also with type 3c lacerations, the IAS is repaired before the EAS and is described next.
With fourth-degree laceration repairs, both torn edges of the rectal mucosa are reapproximated first (see Fig. 27-13). Starting at a point 1 cm proximal to the wound apex, sutures are placed approximately 0.5 cm apart in the rectal muscularis and do not enter the anorectal lumen. Clinicians often use 4–0 delayedabsorbable suture or chromic gut for this running suture line. Some recommend a second reinforcing layer above this (Hale, 2007). The next layer to cover the anorectal mucosa is formed by reapproximation of the IAS.
For reduction of infectious morbidity associated with OASIS, a single dose of antibiotic at the time of repair may be considered (Buppasiri, 2014; Duggal, 2008; Lewicky-Gaupp, 2015; Stock, 2013). A single dose of a second-generation cephalosporin is suitable, or clindamycin for penicillin-allergic women. With OASIS, postoperatively, stool softeners are prescribed for a week, and enemas and suppositories are avoided.
■ Perineal Laceration Care
Initially, locally applied ice packs help reduce swelling and allay discomfort (de Souza Bosco Paiva, 2016). In subsequent days, warm sitz baths aid comfort and hygiene. Additionally, a small squirt bottle of warm water can cleanse the site after voiding or stooling. Analgesics containing codeine provide considerable relief. For lesser degrees of discomfort, nonsteroidal antiinflammatory drugs can be given. Because pain may instead signal a large vulvar, paravaginal, or ischiorectal fossa hematoma or perineal cellulitis, these sites should be examined carefully if pain is severe or persistent. Management of these complications is discussed in Chapters 37 and 42 (pp. 657 and 740). In addition to pain, urinary retention may complicate episiotomy recovery, and management is described in Chapter 36 (p. 643).
For those with second-degree lacerations or OASIS, intercourse is usually proscribed until after the first puerperal visit. Compared with women with intact perineum, those with perineal trauma show higher rates of delayed intercourse at 3 and 6 months, but not at 1 year (McDonald, 2015; Rådestad, 2008; Signorello, 2001)













Nhận xét
Đăng nhận xét