Chapter 37. Puerperal Infection
BS. Nguyễn Hồng Anh
Women are susceptible to several potentially serious complications during the fourth trimester. Many of these conditions are encountered during pregnancy, and others are unique to the puerperium. Historically, infection was the most important source of postpartum maternal morbidity and mortality, since emphasized by the studies of Semmelweis and Lister (Kadar, 2021). Puerperal infections include pelvic infections, mastitis, and breast abscesses. Discussed in their respective chapters, cardiovascular disease (Chap. 52, p. 915), venous thromboembolism (Chap. 55, p. 980), and hemorrhage (Chap. 42, p. 731) currently are leading noninfectious puerperal complications (Callaghan, 2012; Creanga, 2017). However, the incidence of postpartum hospitalization due to sepsis is rising. Other puerperal issues and their management are discussed in Chapter 36.
PUERPERAL PELVIC INFECTIONS
Traditionally, the term puerperal infection describes any bacterial infection of the genital tract after delivery. Infection, preeclampsia, and obstetrical hemorrhage formed the lethal triad of maternal death causes before and during the 20th century. Fortunately, maternal mortality from puerperal infection is uncommon because of effective antibiotics. Creanga and associates (2017) reported results from the Pregnancy Mortality Surveillance System, which contained 4693 pregnancy-related maternal deaths in the United States from 2006 through 2010. Infection caused 13.6 percent of the deaths and was the second leading etiology. In an analysis of the North Carolina population, Berg and colleagues (2005) reported that 40 percent of infection-related maternal deaths were preventable.
■ Puerperal Fever
Several infective and noninfective factors cause puerperal fever defined by a temperature of 38.0°C (100.4°F) or higher. Using this conservative definition, Filker and Monit (1979) reported that only approximately 20 percent of women febrile within the first 24 hours after vaginal delivery were subsequently diagnosed with pelvic infection. Tis value was 70 percent in those undergoing
cesarean delivery. Most persistent fevers after childbirth are caused by genital tract infection. Of note, spiking fevers ≥39°C within the first 24 hours postpartum may be associated with virulent pelvic
infection caused by group A streptococcus (p. 651). Other sources of puerperal fever include breast engorgement, urinary infections, episiotomy and abdominal incisions, perineal lacerations, and postcesarean respiratory complications. Approximately 15 percent of women who do not breast-
feed develop fever from breast engorgement. “Breast fever” rarely exceeds 39°C in the first few postpartum days and usually lasts <24 hours. The incidence of fever is lower in breastfeeding women (Chap. 36, p. 642). Postpartum urinary infections are uncommon because of the normal diuresis. Acute pyelonephritis has a variable clinical picture. The first sign of renal infection may be fever, followed later by costovertebral angle tenderness, nausea, and vomiting. Atelectasis following general anesthesia for cesarean delivery is caused by hypoventilation and is best prevented by coughing and deep breathing on a fixed schedule following surgery. Fever with atelectasis is due to infection triggered by proliferation of normal fora distal to obstructing mucus plugs.
■ Uterine Infection
Postpartum uterine infection has been called endometritis, endomyometritis, and endoparametritis. Following vaginal delivery, infection involves not only the decidua but also the myometrium and parametrial tissues. Infection after cesarean delivery is essentially a surgical site infection involving the incised myometrium. In either case, we prefer the inclusive term metritis with pelvic cellulitis.
Predisposing Factors
Route of delivery is the single most significant risk factor for the development of uterine infection (Boggess, 2017; Moulton, 2018). In women undergoing cesarean delivery, rehospitalization rates for wound complications and metritis are higher than those with a vaginal birth (Axelsson, 2018; Fein, 2019). In the French Confidential Enquiry on Maternal Deaths, DeneuxTaraux and coworkers (2006) cited a nearly 25-fold greater infection-related mortality rate with cesarean delivery. With vaginal delivery, women delivered at Parkland Hospital have a 1- to 2-percent incidence of metritis. This rate rises to 5 to 6 percent in those with ruptured membranes, prolonged labor, and multiple cervical examinations. If intrapartum chorioamnionitis is present, the risk of persistent uterine infection has exceeded 13 percent (DeNoble, 2019; Maberry, 1991).
These figures are similar to those reported by the Maternal Fetal Medicine Units Network from a cohort of more than 115,000 women in whom the overall pelvic infection rate approximated 5 percent (Grobman, 2015).
With operative vaginal delivery, women carry higher risks for metritis and perineal infections than spontaneous birth (Mohamed-Ahmed, 2019). Notably, these deliveries are often implemented in the context of prolonged labor, which is a known metritis risk. One study of 3500 such women showed
that those receiving a single dose of prophylactic antibiotics had a lower maternal infection rate (Knight, 2019). Infections include those of the urinary tract, uterus, or perineal wound. Labor lengths were not presented, but in approximately 50 percent of participants, “failure to progress” was the operative vaginal delivery indication. Moreover, 10 percent of women had rupture of membranes lengths between 24 and 48 hours. The contribution of these, rather than the delivery mode, will
require further investigation before this prophylaxis is widely adopted solely for the indication of operative vaginal delivery. With cesarean delivery, hysterotomy is associated with significant infectious morbidity. In the 1970s, pelvic infection complicated 50 percent of cesarean deliveries at Parkland Hospital. Fortunately, antimicrobial prophylaxis has done more to decrease the incidence and severity of postcesarean infections than any other intervention in the past 30 years. Thus, a single dose of perioperative antibiotics is recommended for all women undergoing cesarean delivery (American
College of Obstetricians and Gynecologists, 2018b). Such practices lower the puerperal pelvic infection risk by 65 to 75 percent (Smaill, 2014). β-lactam antimicrobials are superior to other agents, and elements of this prophylaxis are outlined in Chapter 30 (p. 551) (Harris, 2019). Important risk factors for infection following surgery include prolonged labor, membrane rupture, multiple cervical examinations, and internal fetal monitoring. Regardless of delivery route, pelvic infection generally is more frequent in women of lower socioeconomic status (Maharaj, 2007). Except in extreme cases usually not seen in developed countries, it is uncommon that anemia or poor nutrition predispose to infection. Bacterial colonization of the lower genital tract with certain microorganisms—for example, group B streptococcus, Chlamydia trachomatis, Mycoplasma hominis, Ureaplasma urealyticum, and Gardnerella vaginalis— has been associated with an increased postpartum infection risk (Andrews, 1995; Jacobsson, 2002; Watts, 1990). Other factors associated with a greater risk include general anesthesia, significant hysterotomy extension, young maternal age, nulliparity, prolonged labor induction, chorioamnionitis, obesity, and meconium-stained amnionic fluid (Acosta, 2012; Leth, 2011; Patel, 2019; Siriwachirachai, 2014).
Microbiology
Common Pathogens. Most pelvic infections are caused by bacteria indigenous to the genital tract and listed in Table 37-1. Most of these infections are polymicrobial, which enhances bacterial synergy. Other factors that promote virulence are hematomas and devitalized tissue. Although the cervix and vagina routinely harbor bacteria, the uterine cavity is usually sterile before rupture of the amnionic sac. As the consequence of labor and delivery and associated manipulations, anaerobic and aerobic bacteria contaminate the amnionic fluid and uterus.
TABLE 37-1. Bacteria Commonly Responsible for
Female Genital Infections
Aerobes
Gram-positive cocci: group A, B, and D streptococci,
enterococcus, Staphylococcus aureus, Staphylococcus
epidermidis
Gram-negative bacteria: Escherichia coli, Klebsiella,
Proteus spp.
Gram-variable—Gardnerella vaginalis
Others
Mycoplasma spp., Chlamydia trachomatis, Neisseria
gonorrhoeae
Anaerobes
Cocci: Peptostreptococcus, Peptococcus spp.
Others: Clostridium, Bacteroides, Fusobacterium,
Mobiluncus spp.
Puerperal Infection 651
CHAPTER 37
Prior to the routine use of antimicrobial prophylaxis, Gilstrap and Cunningham (1979) cultured amnionic fluid obtained at cesarean delivery from women in labor with membranes ruptured more than 6 hours. All had bacterial growth, and an average of 2.5 organisms was identified from each specimen. Anaerobic and aerobic organisms were found in 63 percent, anaerobes alone in 30 percent, and aerobes alone in only 7 percent. Anaerobes included Peptostreptococcus and Peptococcus species in 45 percent, Bacteroides species in 9 percent, and Clostridium species in 3 percent. Clostridial species rarely cause puerperal infections, but infections can be severe in those cases (Herrera, 2016). Aerobes included Enterococcus in 14 percent, group B Streptococcus in 8 percent, and Escherichia coli in 9 percent of isolates. Sherman and associates (1999) later showed that bacterial isolates at cesarean delivery correlated with those taken from women with metritis at 3 days postpartum. Group B streptococci, E coli, and enterococci are some of the more common blood culture isolates with metritis (Cape, 2013; O’Higgins, 2014). In the past 15 years, skin and soft-tissue infections due to methicillin- resistant Staphylococcus aureus (MRSA) have become prevalent (Chap. 67, p. 1196). However, MRSA is more commonly implicated in abdominal and perineal incisional infections and less often in puerperal metritis (Anderson, 2007; Patel, 2007). Group A β-hemolytic streptococcal infections have a reported incidence of 1 in 1220 births (Rottenstreich, 2019). During the past 25 years, group A streptococcus has been reported to cause a toxic shock–like syndrome and life-threatening infection
(Donders, 2021; Gustafson, 2017; Shinar, 2016). In reviews by Crum (2002) and Udagawa (1999), group A streptococcal infections manifested before, during, or within 12 hours of delivery. Affected women had a maternal mortality rate of almost 90 percent, and the fetal mortality rate exceeded 50 percent.
The role of other organisms in the etiology of these infections is unclear. Observations of Chaim and coworkers (2003) suggest that heavy cervical colonization of U urealyticum may contribute to metritis development. To counter this potential pathogen, azithromycin-based extended spectrum antibiotic prophylaxis reduces postoperative cesarean delivery infections from 12 to 6 percent compared with β-lactam agents alone (Harper, 2017; ita, 2016). Chlamydial infections have been implicated in late-onset, indolent metritis (Ismail, 1985). Last, Jacobsson and colleagues (2002) reported a threefold higher risk of puerperal infection in a group of Swedish women in whom bacterial vaginosis was identified in early pregnancy (Chap. 68, p. 1216).
Bacterial Cultures. Routine genital tract cultures obtained before treatment serve little clinical use and add significant costs. Women with a temperature >102°F are more likely to have bacteremia (Easter, 2017). Even so, routine blood cultures seldom modify care. In two studies done before perioperative prophylaxis was used, blood cultures were positive in 13 percent of women with postcesarean metritis at Parkland Hospital and in 24 percent of those at Los Angeles County Hospital (Cunningham, 1978; DiZerega, 1979). In a later Finnish study, bacteremia was identified in only 5 percent of women with puerperal sepsis (Kankuri, 2003). Exceptions might be women with exceedingly high temperature spikes that may signify virulent infection with group A streptococci (Chap. 50, p. 890).
Pathogenesis and Clinical Course Puerperal infection following vaginal delivery primarily
involves the placental implantation site, decidua and adjacent myometrium, or cervicovaginal lacerations. The pathogenesis of uterine infection following cesarean delivery is that of an
infected surgical incision. Bacteria that colonize the cervix and vagina gain access to the uterus during labor. Postpartum, they invade devitalized uterine tissue. Parametrial cellulitis follows with infection of the pelvic retroperitoneal fibroareolar connective tissue. With early treatment, infection is contained within the parametrial and paravaginal tissue, but it may extend deep into the pelvis.
Fever is the most important criterion for the diagnosis of postpartum metritis. Intuitively, the degree of fever is believed proportional to the extent of infection and sepsis. Temperatures usually are 38 to 39°C, and temperatures >39°C suggest bacteremia or endotoxemia (Easter, 2017; Sufredini, 1989).
Women usually complain of abdominal pain, and parametrial tenderness is elicited on abdominal and bimanual examination. Leukocytosis may range from 15,000 to 30,000 cells/μL, but recall that cesarean delivery itself raises the leukocyte count. Although an offensive odor can develop, many women have foul-smelling lochia without evidence for infection, and vice versa. Some other infections, notably those caused by group A β-hemolytic streptococci, may be associated with scant, odorless lochia (Anderson, 2014).
Treatment
If metritis develops following vaginal delivery, treatment with an oral or intramuscular antimicrobial agent may be sufficient (Meaney-Delman, 2015). For moderate to severe infections, however, intravenous therapy with a broad-spectrum antibiotic regimen is indicated. Improvement occurs in 48 to 72 hours in nearly 90 percent of women treated with one of several regimens discussed below. Persistent fever after this interval mandates a careful search for causes of refractory pelvic infection. These include a parametrial phlegmon—an area of intense cellulitis; an abdominal incisional or pelvic abscess or infected hematoma; and septic pelvic thrombophlebitis. In our experience, persistent fever is seldom due to antimicrobial-resistant bacteria or due to drug side effects. The woman may be discharged home after she has been afebrile for at least 24 hours, and further oral antimicrobial therapy is not needed (Mackeen, 2015).
Choice of Antimicrobials. Terapy is empirical and initial treatment is directed against the mixed fora shown in Table 37-1. For infections following vaginal delivery, as many as 90 percent of women respond to regimens such as ampicillin plus gentamicin. In contrast, anaerobic coverage is included for infections following cesarean delivery (Table 37-2). In 1979, DiZerega and coworkers compared the effectiveness of clindamycin plus gentamicin with that of penicillin G plus gentamicin for treatment of pelvic infections following cesarean delivery. Women given the clindamycin-gentamicin regimen had a 95-percent response rate, and this regimen is still considered by most to be the standard by which others are measured (Mackeen, 2015). Despite this standard therapy, enterococcal cultures may be persistently positive. The addition of ampicillin, either initially or following no response after 48 to 72 hours, targets enterococci (Brumeld, 2000).
Many authorities recommend periodical monitoring of serum gentamicin levels. At Parkland Hospital, we do not routinely do so if a woman has adequate renal function, which is evidenced by a normal serum creatinine level. Once-daily dosing versus multiple dosing with gentamicin provides adequate serum levels, and either method has similar cure rates (Livingston, 2003). In the event of diminished glomerular filtration, some recommend a combination of clindamycin and a second-generation cephalosporin, because of potential nephrotoxicity and ototoxicity with gentamicin. Instead, others recommend a combination of clindamycin and aztreonam—the latter is a monobactam compound with activity similar to the aminoglycosides. The spectra of β-lactam antimicrobials include activity
against many anaerobic pathogens. Some examples include cephalosporins such as cefoxitin, cefotetan, cefotaxime, and cef- triaxone. Extended-spectrum penicillins are piperacillin, ticarcillin, and mezlocillin. β-lactam antimicrobials are inherently safe and, except for allergic reactions, are free of major toxicity. Te β-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam have been combined with ampicillin, amoxicillin, ticarcillin, and piperacillin to extend their spectra. Metronidazole has superior in vitro activity against most anaerobes. This agent given with ampicillin and an aminoglycoside provides coverage against most organisms encountered in serious pelvic infections. It is also used second line to treat some cases of Clostridioides difcile colitis.
Imipenem and similar antimicrobials are in the carbapenem family. These offer broad-spectrum coverage against most organisms associated with metritis. Imipenem coupled with cilastatin inhibits the antibiotic’s renal metabolism. Because of imipenem’s higher cost, it is reasonable from both a medical and an economic standpoint to reserve this drug for serious nonobstetrical infections. Vancomycin is a glycopeptide antimicrobial active against gram-positive bacteria. It is used in lieu of β-lactam therapy for a patient with a type 1 allergic reaction and given for suspected infections due to S aureus and to treat C difcile colitis (Chap. 57, p. 1019).
Perioperative Prophylaxis
The use of periprocedural techniques for infection prevention is common in obstetrics (Table 37-3). Numerous studies show that prophylactic antibiotics at the time of cesarean delivery reduce wound and postpartum pelvic infection rates (Carter, 2017; Smaill, 2014). The observed benefit applies to both elective and nonelective cesarean delivery. As noted from preliminary data, antimicrobials may decrease pelvic infection rates after operative vaginal delivery (p. 650). However, data are insufficient to suggest prophylactic antimicrobials lower infection rates after spontaneous vaginal delivery, repair of all episiotomies, or manual extraction of the placenta (Bonet 2017a,b; Chongsomchai, 2014). The American College of Obstetricians and Gynecologists (2018a) concluded that a single antibiotic dose with third- and fourth-degree perineal laceration is reasonable and has evidenced-based support. Single-dose prophylaxis with a 2-g dose of a first- generation cephalosporin is ideal. This regimen has similar efficacy of broad-spectrum agents or multiple-dose regimens (American College of Obstetricians and Gynecologists, 2018b). For obese women, evidence supports a 3-g dose of cefazolin to reach optimal tissue concentrations (Swank, 2015). Some evidence supports addition of azithromycin to lower postcesarean uterine infection rates (Markwei, 2021; Pierce, 2021; ita, 2016). Women colonized with MRSA are given vancomycin
TABLE 37-2. Antimicrobial Regimens for Pelvic Infections Following Cesarean Delivery
| Regimen Clindamycin + gentamicin | Comments “Gold standard,” 90–97% efficacy, once-daily gentamicin dosing acceptable Plus Ampicillin added to regimen with sepsis or suspected enterococcal infection Gentamicin substitute for renal insufficiency Piperacillin, piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanate Cefotetan, cefoxitin, cefotaxime, ceftriaxone |
| Clindamycin + aztreonam Extended-spectrum penicillins Cephalosporins | |
| Vancomycin | Added to other regimens for suspected Staphylococcus aureus infections |
ampicillin + gentamicin
Metronidazole has excellent anaerobic coverage
Carbapenems Imipenem/cilastatin, meropenem, ertapenem; all reserved for special indications
TABLE 37-3. Various Prophylactic Methods for Decreasing Pelvic and Wound Infection Rates Following Delivery
| Route Routine delivery Episiotomy Operative vaginal delivery Cesarean delivery Cesarean delivery | Method Peripartum antimicrobials Perioperative prophylaxis Peripartum antimicrobials Perioperative antimicrobial prophylaxis Skin preparation | Study Results Limited evidence, may reduce risk (Bonet, 2017a) Insufficient evidence (Bonet, 2017b) Limited evidence, may reduce risk (Knight, 2019) Decreased 70–80% (Carter, 2017; Smaill, 2014) Decreased incidence (Hadiati, 2018) |
in addition to a cephalosporin (Chap. 67, p. 1196). It is controversial whether the infection rate is reduced further if the antimicrobial is given before the skin incision compared with after umbilical cord clamping (Baaqeel, 2013; Macones, 2012; Sun, 2013; Ward, 2016). The American College of Obstetricians and Gynecologists (2018b) has concluded that the evidence favors predelivery administration.
Most women with a stated allergy to penicillin are not prone to developing anaphylaxis. Without a history of anaphylaxis, most of these women can safely be given a cephalosporin (Chap. 30, p. 551). If not, then vancomycin is given along with clindamycin and gentamicin (American College of Obstetricians and Gynecologists, 2018b).
Preoperative abdominal skin preparation decreases the risk for pelvic and wound infections (Hadiati, 2018). Skin preparation with chlorhexidine-alcohol is superior to iodine-alcohol for preventing surgical-site infections (Tuuli, 2016). Additive beneficial effects may be gained by preoperative vaginal cleansing with povidone-iodine rinse or application of metronidazole gel (Caissutti, 2017; Felder, 2019; Haas, 2018).
Other Methods of Prophylaxis. Several studies have addressed the value of prenatal cervicovaginal cultures. These are obtained in the hope of identifying pathogens that might be eradicated to lower incidences of preterm labor, chorioamnionitis, and puerperal infections. Unfortunately, treatment of asymptomatic vaginal infections does not prevent these complications. For asymptomatic bacterial vaginosis, Carey and associates (2000) reported no beneficial effects for women treated. For asymptomatic Trichomonas vaginalis infection, a similar postpartum infection rate was found in women treated in the second trimester compared with placebo-treated women (Klebano, 2001).
Technical maneuvers done to alter the postcesarean infection rate have been studied (Chap. 30, p. 551). Allowing the placenta to separate spontaneously and exteriorizing the uterus to close the hysterotomy may reduce the infection risk (JacobsJokhan, 2004; Lasley, 1997). However, changing gloves after placental delivery, cleaning the intrauterine cavity, and dilating the lower segment and cervix do not alter the infection rate (Atkinson, 1996; Eke, 2019; Liabsuetrakul, 2018). No differences were found in postoperative infection rates when singleand two-layer uterine closures were compared (Hauth, 1992). Similarly, infection rates are not affected by closure versus nonclosure of the peritoneum (Bamigboye, 2014; Tulandi, 2003). Importantly, closure of subcutaneous tissue in obese women does not lower the wound infection rate, but it does decrease the wound separation incidence (Chelmow, 2004). Similarly, skin closure with staples versus suture has a greater incidence of noninfectious skin separation (Mackeen, 2012; Tuuli, 2011).
■ Complications of Uterine and Pelvic Infections
Metritis responds to antimicrobial treatment within 48 to 72 hours in more than 90 percent of women. In some of the remainder, any of several complications may arise. These include wound infection, complex pelvic infection such as a phlegmon or an abscess, and septic pelvic thrombophlebitis (Brown, 1999; Jaiyeoba, 2012). As with other aspects of puerperal infections, the incidence and severity of these complications are reduced by perioperative antimicrobial prophylaxis.
■ Abdominal Incisional Infections
Wound infection is a common cause of persistent fever in women treated for metritis. Incisional infection risk factors include obesity, diabetes, corticosteroid therapy, immunosuppression, anemia, hypertension, and hematoma formation from inadequate hemostasis. If prophylactic antimicrobials are given, the incidence of wound infection following cesarean delivery ranges from 2 to 10 percent depending on risk factors (Andrews, 2003; Chaim, 2000). From our experiences at Parkland Hospital, the incidence is closer to 2 percent, but this risk rises with increasing body mass (Hussamy, 2018). Incisional abscesses that develop following cesarean delivery usually cause persistent fever or fever that begins on approximately the fourth day. The wound is erythematous and drains pus. Organisms that cause wound infections are generally the same as those isolated from amnionic fluid at cesarean delivery, however, hospital-acquired pathogens also may be causative.
Treatment includes antimicrobials and surgical drainage and debridement of devitalized tissue. This typically requires spinal analgesia or general anesthesia. The fascia is carefully inspected to document integrity. Local wound care thereafter is completed twice daily. Before each dressing change, procedural analgesia is tailored to wound size and location, and oral, intramuscular, or intravenous dosage routes are suitable. Topical lidocaine also may be added. Necrotic tissue is removed, and the wound is repacked with moist gauze. At 4 to 6 days, healthy granulation tissue is typically present, and secondary en bloc closure of the open layers can usually be accomplished ( Wechter, 2005). As shown in Figure 37-1, a polypropylene or
FIGURE 37-1 Secondary abdominal wound closure technique.
(Reproduced with permission from Worley KC: Postoperative complications. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al [eds]:
Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York,
NY: McGraw Hill; 2017).
FIGURE 37-2 Theoretical effects of negative-pressure wound therapy include macro- and microdeformation, removal of tissue fluid, and creation of a warm and moist environment. As shown in the inset, tissue fluid is drawn out by suction tubing. It travels through the porous sponge dressing that fills the wound and into an adjacent collection canister. As healing progresses, a layer of granulation tissue (red) forms at the wound-sponge interface. (Reproduced with permission from Cunningham FG: Surgical instruments. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al [eds]: Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
nylon suture of appropriate gauge enters 2 to 3 cm from one wound edge. It crosses the wound to incorporate the full wound thickness and emerges 3 cm from the other wound edge. These are placed in series to close the opening. In most cases, sutures may be removed on postprocedural day 10.
Vacuum-Assisted Wound Closure
This system promotes healing by applying negative pressure to the wound (Fig. 37-2). The technique is variably referred to as vacuum-assisted closure (VAC), topical negative pressure (TNP), and negative-pressure wound therapy (NPWT). Negative-pressure systems are used for open wounds after infection or for prophylaxis against wound disruption. Mouës and colleagues (2011) confirmed that NPWT enhances blood flow to the wound, promotes angiogenesis, induces cellular proliferation, and shrinks wound size. Several systems are available and widely accepted, despite meager formal evidence for clinical efficacy. Open abdominal wounds once infection has cleared or an “open surgical abdomen” is a major indication for NPWT.
Vacuum therapy is the most efficient method of temporary abdominal closure for patients with open abdominal wounds (Bruhin, 2014; Quyn, 2012). However, no trials have compared vacuum-assisted wound closure with conventional wound care after cesarean delivery. Also, these devices are used for closure of perineal wounds resulting from infected episiotomies, hematomas, or abscesses (Aviki, 2015). Very few randomized trials have compared vacuum-assisted wound closure with conventional
wound care (Kawakita, 2021; Yu, 2018).
Prophylaxis. Negative-pressure devices are also marketed to prevent wound infections in incisions closed to heal by primary intention. Such prophylactic use in 441 obese women undergoing cesarean delivery has been evaluated in two large randomized trials. Hussamy and associates (2019) found in obese women that the incidence of wound morbidity was similar in the standard dressing group compared with the NPWT group (Table 37-4). The second trial was a multicenter study from Denmark of 876 obese women (Hyldig, 2019). In the NPWT group, the surgical site infection rate was 4.6 percent compared with 9.2 percent in the group of women treated with standard dressing. Similarly, a systematic review found use of prophylactic NPWT reduced wound complications (Yu, 2018). The cost effectiveness of these systems is inconclusive in multiple studies (Echebiri, 2015; Lewis, 2014). Smid and coworkers (2017) questioned the effectiveness of such therapy. Because of these uncertainties, we agree with Fuuli (2019) that routine use of prophylactic NPWT needs more evaluation before its widespread acceptance.
Fascial Dehiscence
This separation of the fascial layer is a serious complication, and bowel evisceration can be comorbid. Wound infection and obesity are prominent risk factors (Poole, 1985; Subramaniam, 2014). For example, McNeeley and associates (1998) reported a fascial dehiscence rate of approximately 1 per 300 operations in almost 9000 women undergoing cesarean delivery. Two thirds of the 27 fascial dehiscences in this study were associated with concurrent fascial infection and tissue necrosis. Fascial dehiscence generally presents within the first 7 to 10 postoperative days. Superficial disruption of the subcutaneous layer and extensive leakage of peritoneal fluid or purulent drainage are indicative. In unclear cases, CT scanning may be elected preoperatively, if obtained expeditiously. Dehiscence is a surgical emergency. If abdominal contents have eviscerated, sterile towels or gauze soaked in saline can be used early to cover and gently replace bowel or omentum. Broadspectrum antibiotics are generally recommended to minimize ensuing peritonitis.
Given the high mortality risk associated with fascial dehiscence and bowel evisceration, examination under anesthesia to estimate the extent of separation is often warranted. Surgery aims to assess
bowel health, debride necrotic wound tissue, and close the fascia, if possible. For closure, an interrupted mass closure using a no. 2 permanent suture is recommended typically (Fig. 37-3). In cases
with infection, the subcutaneous layers are left to close secondarily. General surgery consultation is considered if bowel ischemia or difficult fascial closure is anticipated.
■ Necrotizing Fasciitis
This uncommon severe wound infection is associated with high mortality rates. In obstetrics, necrotizing fasciitis may involve abdominal incisions, or it may complicate episiotomy or other
perineal lacerations. As the name implies, tissue necrosis is significant. Of the risk factors for fasciitis summarized by Owen and Andrews (1994), diabetes, obesity, and hypertension are increasingly common in gravidas. Like pelvic infections, this wound complication usually is polymicrobial and caused by
FIGURE 37-3 Mass closure: each stitch is placed 1.5 to 2 cm from the wound edge (A) and incorporates the peritoneum, rectus muscle, and rectus sheath (B). Stitches are spaced 1 cm apart along the length of the incision. A.R.S. = anterior rectus sheath; P.R.S. = posterior rectus sheath; SubQ = subcutaneous layer. (Reproduced with permission from Cundiff GW: Incisions and closures. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al [eds]: Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
organisms that make up the normal vaginal fora. In some cases, however, infection is caused by a single virulent bacterial species such as group A β-hemolytic streptococcus (Anderson, 2014; Rimawi, 2012). Occasionally, rarely encountered pathogens cause necrotizing infections (Chong, 2016; Swartz, 2004).
Goepfert and coworkers (1997) described nine cases of necrotizing fasciitis in more than 5000 cesarean deliveries. The infection was fatal in two women—one with metastatic breast cancer and the other with sepsis. In another report, Schorge and colleagues (1998) described five women with fasciitis following cesarean delivery. None of these women had predisposing risk factors, and none died.
Infection may involve skin, superficial and deep subcutaneous tissues, and any of the abdominopelvic fascial layers. In some cases, muscle also is involved—myofasciitis. Most of these necrotizing infections do not cause symptoms until 3 to 5 days after delivery. Some virulent infections develop earlier. Clinical findings vary, and it is frequently difficult to differentiate more innocuous superficial wound infections from a deep fascial one. If myofasciitis progresses, the woman may become ill from septicemia. A high index of suspicion, with surgical exploration if the diagnosis is uncertain, may be lifesaving (Goh, 2014). We aggressively pursue early exploration (Chap. 50, p. 890). Successful treatment of necrotizing soft-tissue infections involves early diagnosis, source control by surgical debridement, antimicrobials, and intensive care (Gallup, 2002;
Goh, 2014; Society for Maternal-Fetal Medicine, 2019). Surgery includes thorough debridement of all infected tissue, leaving wide margins of healthy bleeding tissue. This may include extensive abdominal or vulvar debridement and excision of abdominal, thigh, or buttock fascia. Death is virtually universal without surgical treatment, and rates approach 50 percent even if exhaustive debridement is performed (Johnson, 2020). With substantial resection, synthetic mesh may ultimately be required later to close the fascial incision once infection is cleared (Gallup, 2002; McNeeley, 1998).
■ Peritonitis and Adnexal Abscesses
Following cesarean delivery, peritonitis is infrequent. It almost always is preceded by metritis, especially cases with uterine incisional necrosis and dehiscence. However, it may stem from a ruptured adnexal abscess or an inadvertent intraoperative bowel injury. Perforative appendicitis also can cause peritonitis (Chap. 57, p. 1024). In these cases, prompt surgical treatment is usually indicated.
After vaginal delivery, peritonitis is rarely encountered, and many such cases are due to virulent strains of group A β-hemolytic streptococci or similar organisms. Importantly, abdominal rigidity may not be prominent with puerperal peritonitis because of physiological abdominal wall laxity from pregnancy. Pain may be severe, but frequently, the first symptoms of peritonitis are those of adynamic ileus. Marked bowel distention may develop, which is unusual after vaginal birth. Normally, if the infection begins in an intact uterus and extends into the peritoneum, antimicrobial treatment alone sutes. An ovarian abscess rarely develops in the puerperium. These are presumably caused by bacterial invasion through an opening in the ovarian capsule (Wetchler, 1985). The abscess is usually unilateral, and women typically present 1 to 2 weeks after delivery. Rupture is common, and peritonitis may be severe.
■ Parametrial Phlegmon
For some women in whom metritis develops following cesarean delivery, parametrial cellulitis is intense and forms an area of induration—a phlegmon—within the leaves of the broad ligament (Fig. 37-4). These infections are considered when fever persists longer than 72 hours despite intravenous antimicrobial therapy (Brown, 1999; DePalma, 1982). Phlegmons are usually unilateral, and they frequently are limited to the parametrium at the base of the broad ligament. If the inflammatory reaction is more intense, cellulitis extends along natural lines of cleavage. The most common form of extension is laterally along the broad ligament, with a tendency to extend to the pelvic sidewall. Occasionally, posterior extension may involve the rectovaginal septum, producing a firm mass posterior to the cervix. In most women, clinical improvement follows continued treatment
with a broad-spectrum antimicrobial regimen. Typically, fever resolves in 5 to 7 days. Severe cellulitis of the uterine incision may ultimately lead to necrosis and separation (Treszezamsky, 2011). Extrusion of purulent material causes intraabdominal abscess formation and peritonitis as described above. Surgery is reserved for women in whom uterine incisional necrosis is suspected. For most, hysterectomy and surgical debridement are needed. These cases are difficult because the cervix and lower uterine segment are involved with an intense inflammatory process that extends to the pelvic sidewall. The adnexa are seldom involved, and one or both ovaries can usually be conserved. Blood loss is often appreciable, and transfusion is common.
Imaging Studies
Persistent puerperal infections can be evaluated using computed tomography (CT) or magnetic resonance (MR) imaging (Wang, 2020). Brown and associates (1991) used CT imaging in women
in whom pelvic infection was refractory to antimicrobial therapy given for 5 days. They found at least one abnormal radiological finding in 75 percent of these women, and most were nonsurgical lesions. Fishel Bartal and colleagues (2018) reported abnormal CT findings in almost 60 percent of women with refractory fever persisting >3 days. Pelvic fluid collections were seen in 22 percent, and surgical intervention was prompted in 8 percent. Tus, imaging can be used to dissuade surgical exploration in most cases.
FIGURE 37-4 Left-sided parametrial phlegmon: cellulitis causes induration in the parametrium adjacent to the hysterotomy incision. (Reproduced with permission from Worley KC: Postoperative complications. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al [eds]: Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017).
Puerperal Infection 657
CHAPTER 37
Uterine incisional dehiscence such as shown in Figure 37-5 can sometimes be confirmed based on CT images. These findings must be interpreted within the clinical context because apparent uterine incisional defects thought to represent edema can be seen even after uncomplicated cesarean delivery. Shown in Figure 37-6 is a necrotic hysterotomy incision, which had leaked into the peritoneal cavity.
Occasionally, a parametrial phlegmon may suppurate, forming a fuctuant broad ligament abscess that may point above the inguinal ligament. These abscesses can dissect anteriorly and be amenable to CT directed needle drainage. A psoas abscess is rare, and despite antimicrobial therapy, percutaneous drainage may be required to effectively treat it (Shahabi, 2002; Swanson, 2008). If a phlegmon involving the rectovaginal septum suppurates, surgical drainage is easily effected by colpotomy. Absorption of the induration may require several days to weeks.
■ Septic Pelvic Thrombophlebitis
Suppurative thrombophlebitis was a frequent complication in the preantibiotic era, and septic embolization was common. However, with the advent of antimicrobial therapy, the mortality rate and
need for surgical therapy for these infections diminished. Septic phlebitis arises as an extension along venous routes and may cause thrombosis. Lymphangitis often coexists. The ovarian veins may then become involved because they drain the upper uterus and therefore the placental implantation site. Te experiences of Witlin and Sibai (1995) and Brown and coworkers (1999) suggest that puerperal septic thrombophlebitis is likely to involve one or both ovarian venous plexuses. In a fourth of women, the clot extends into the inferior vena cava and occasionally to the renal vein. The incidence of septic phlebitis varies in several reports. In a 5-year survey of 45,000 women who were delivered at Parkland Hospital, Brown and workers (1999) found an incidence of 1 case per 9000 vaginal births and 1 per 800 cesarean deliveries. In a cohort of 16,650 women undergoing primary cesarean delivery, Rouse and coworkers (2004) reported an incidence of 1 case per 400 surgeries. Incidences approximated 1 per 175 cesarean deliveries if chorioamnionitis was antecedent, but only 1 per 500 if there was no intrapartum infection.
Except for chills and occasional lower quadrant pain, women with septic thrombophlebitis usually lack symptoms (Wouterlood, 2021). The diagnosis can be confirmed by pelvic CT or MR imaging (Fig. 37-7). Using either, Brown and coworkers (1999) ound that 20 percent of 69 women with persistent fever following >5 days of antimicrobial therapy for metritis had septic pelvic thrombophlebitis. In a later study, Fischel Bartal and associates (2018) reported that 6 percent of women with refractory fever ≥3 days had septic phlebitis. These women normally have symptomatic improvement with antimicrobial treatment, however, they continue to have fever. Treatment with heparin is controversial. In a randomized study of 14 women, the addition of heparin to antimicrobial therapy for septic pelvic thrombophlebitis did not hasten recovery or improve outcome (Brown, 1999). We and others are of the opinion that heparin is unnecessary (Witlin, 1995). Others, however, continue to recommend anticoagulation (Klima, 2008; Lenz, 2017). Certainly, no evidence supports long-term anticoagulation (Brown, 2018).
■ Perineal Infections
Episiotomy infections are uncommon, because the operation is now performed less frequently (American College of Obstetricians and Gynecologists, 2018a; Dillon, 2019). Reasons for this are discussed in Chapter 27 (p. 510). In an older study, Owen and Hauth (1990) described only 10 episiotomy infections in 20,000 women delivered vaginally. With infection, however, dehiscence is a concern. Ramin and colleagues (1992) reported an episiotomy dehiscence rate of 0.5 percent at Parkland Hospital, and 80 percent of these were infected. Uygur and associates (2004) reported a 1-percent dehiscence rate and attributed two thirds to infection. When the anal sphincter is disrupted at delivery, the subsequent infection rate is higher and is likely influenced by intrapartum antimicrobial treatment (Buppasiri, 2014; Stock, 2013).
FIGURE 37-5 Pelvic computed tomography scan showing necrosis of the uterine incision with gas in the myometrium (arrows). A large abscess (a) fills the right parametrium.
FIGURE 37-6 Necrotic hysterotomy infection. Severe cellulitis of the uterine incision resulted in dehiscence with subsequent leakage into the peritoneal cavity. Hysterectomy was required for sufficient debridement of necrotic tissue. (Reproduced with permission from Dr. Denisse Holcomb.)
Lewicky-Gaupp and colleagues (2015) reported a 20-percent infection rate when the sphincter was torn. Goldaber and coworkers (1993) described fourth-degree lacerations in 390 parturients, of whom 5.4 percent had morbidity. In these women, 2.8 percent had infection and dehiscence, 1.8 percent had only dehiscence, and 0.8 percent only infection.
Pathogenesis and Clinical Course
As noted, perineal laceration infection may be complicated by dehiscence. Other factors for separation include coagulation disorders, smoking, and human papillomavirus infection (Ramin, 1994). No data suggest that dehiscence is related to faulty repair.
With infection, local pain and dysuria, with or without urinary retention, are frequent symptoms. Ramin and colleagues (1992) reported that the most common findings were pain in 65 percent, purulent discharge in 65 percent, and fever in 44 percent. In extreme cases, the entire vulva may become edematous, ulcerated, and covered with exudate. Although life-threatening, septic shock or necrotizing fasciitis is rare (p. 654).
Vaginal lacerations may also become infected directly or by extension from the perineum. The epithelium becomes red and swollen and may then become necrotic and slough. Parametrial extension can lead to lymphangitis. Cervical lacerations are seldom noticeably infected, but instead may manifest as metritis. Deep lacerations that extend directly into the base of the broad ligament may become infected and cause lymphangitis, parametritis, and bacteremia.
Treatment
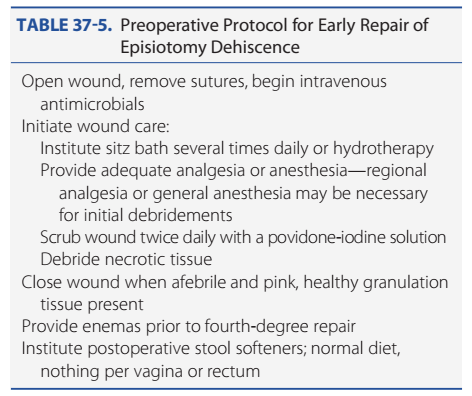
Infected episiotomies are managed similar to other infected surgical wounds. In women with obvious cellulitis but no purulence, close observation and broad-spectrum antimicrobial therapy alone may be appropriate. With purulence, drainage is established, and in most cases, sutures are removed and the infected wound debrided. With dehiscence, local wound care is coupled with intravenous antimicrobials. Hauth and associates (1986) were the first to advocate for early episiotomy repair after infection subsided. Hankins and colleagues (1990) described successful early repair in 94 percent of women, and the average duration from dehiscence to repair was 6 days. The two women with failures developed a pinpoint rectovaginal fistula that was treated with a small rectal flap. Other studies have shown similar high rates with early repair (Ramin, 1992; Uygur, 2004). Before performing early repair, diligent preparation is essential (Table 37-5). Te surgical wound must be properly cleaned and cleared of infection. Once the surface of the wound is free
FIGURE 37-7 Septic ovarian vein thrombosis—contrast-enhanced computed tomography scan: A. Enlarged right ovarian vein filled with low-density thrombus (black arrow). Contrast is seen in ureter (white arrow). R = lower pole, right kidney. B. Coronal image demonstrates enlarged right ovarian vein filled with low-density thrombus (arrows). (B: Reproduced with permission from Dr. April Bailey in Worley KC: Postoperative complications. In Yeomans ER, Hoffman BL, Gilstrap LC III, et al [eds]: Cunningham and Gilstrap’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
TABLE 37-5. Preoperative Protocol for Early Repair of
Episiotomy Dehiscence
Open wound, remove sutures, begin intravenous
antimicrobials
Initiate wound care:
Institute sitz bath several times daily or hydrotherapy
Provide adequate analgesia or anesthesia—regional
analgesia or general anesthesia may be necessary
for initial debridements
Scrub wound twice daily with a povidone-iodine solution
Debride necrotic tissue
Close wound when afebrile and pink, healthy granulation
tissue present
Provide enemas prior to fourth-degree repair
Institute postoperative stool softeners; normal diet,
nothing per vagina or rectum
Puerperal Infection 659
CHAPTER 37
of exudate and covered by pink granulation tissue, secondary repair can be accomplished. Te tissue must be adequately mobilized, with special attention to identify and mobilize the anal sphincter muscle. A tension-free suture line is essential to avoid repeated dehiscence. Secondary closure of the wound is accomplished in layers, as described for primary episiotomy closure (Chap. 27, p. 510). Postoperative care includes local wound care, stool softeners, and nothing per vagina or rectum until healed. Hard stools risk wound disruption, but liquid stool can seep between sutures to reincite infection. Thus, soft formed stools are the goal.
■ Toxic Shock Syndrome
This acute febrile illness with severe multisystem derangement has a case-fatality rate of 10 to 15 percent. Usual findings are fever, headache, mental confusion, diffuse macular erythematous rash, nausea, vomiting, watery diarrhea, and marked hemoconcentration. Renal failure followed by hepatic failure, disseminated intravascular coagulation, and circulatory collapse may progress in rapid sequence. During recovery, the rash-covered areas desquamate. In early investigations of toxic
shock syndrome (SS), Staphylococcus aureus was recovered from almost all aficted persons. Specifically, a staphylococcal exotoxin, termed toxic shock syndrome toxin 1 (TSST-1), was found to cause the clinical manifestations by provoking pro- found endothelial injury. A very small amount of SS-1 can activate T cells to create a “cytokine storm” (Heying, 2007; Que, 2005). Toxic shock syndrome has also been reported with MRSA (Deguchi, 2018).
Subsequent investigations have also implicated virulent group A β-hemolytic streptococcal infection (Anderson, 2014; Rottenstreich, 2019; Shinar, 2016). Heavy colonization or infection is complicated in some cases by streptococcal toxic shock syndrome, which is produced when pyrogenic exotoxin is elaborated. Serotypes M1 and M3 are particularly virulent (Beres, 2004; Okumura, 2004). Last, almost identical findings of toxic shock have been reported in pregnant women with Clostridium sordellii and novyi colonization (Herrera, 2016; Robbie, 2000). Thus, in some cases of toxic shock syndrome, infection is not apparent, and colonization of a mucosal surface is the presumed source (Olp, 2020). At least 10 to 20 percent of pregnant women have vaginal colonization with S aureus. And Clostridium perfringens and sordellii are cultured from 3 to 10 percent of asymptomatic women (Chong, 2016). Tus, it is not surprising that the disease develops in postpartum women when growth of vaginal bacteria is abundant (Chen, 2006; Guerinot, 1982). Delayed diagnosis and treatment are associated with maternal mortality (Schummer, 2002). Crum and colleagues (2002) described a neonatal death following antenatal toxic shock syndrome. Principal therapy is supportive, while allowing reversal of capillary endothelial injury. Antimicrobial therapy includes coverage against staphylococcal and streptococcal species. With evidence of pelvic infection, antimicrobial therapy must also include agents used for polymicrobial infections. Women with these inections may require wound debridement and possibly hysterectomy. Because the toxin is so potent, the mortality rate is correspondingly high (Hotchkiss, 2003).
BREAST INFECTIONS
Parenchymal infection of the mammary glands is a rare antepartum complication, but the postpartum incidence of mastitis approximates 3 percent (Lee, 2010). No evidence supports use of prophylactic measures to prevent breast infection ( Crepinsek, 2012). Risk factors include nursing difficulties,
cracked nipples, and oral antibiotic therapy (Branch-Elliman, 2012; Mediano, 2014).
Symptoms of suppurative mastitis seldom appear before the end of the first week postpartum and usually are not seen until the third or fourth week. Infection almost invariably is unilateral, and marked engorgement usually precedes inflammation. Symptoms include chills or actual rigors, which are soon followed by fever and tachycardia. Pain is severe, and the breast(s) becomes hard and red. Approximately 10 percent of women with mastitis develop an abscess. Detection of fuctuation may be difficult, and sonography is usually diagnostic (Fig. 37-8). Although rare, toxic shock syndrome from mastitis caused by S aureus has been reported (Demey, 1989; Fujiwara, 2001).
FIGURE 37-8 Puerperal mastitis with breast abscess. A. Indurated, erythematous skin overlies the area of right-sided breast infection. B. Sonographic picture of this 5-cm abscess.
■ Etiology
S aureus, especially MRSA, is the most commonly isolated organism in breast infections. Matheson and coworkers (1988) found it in 40 percent of women with mastitis. Other commonly isolated organisms are coagulase-negative staphylococci and viridans streptococci. The immediate source of mastitis-causing organisms is almost always the newborn’s nose and throat. Bacteria enter the breast through the nipple at fissures or small abrasions. The infecting organism can usually be cultured from milk.
At times, suppurative mastitis reaches epidemic levels among nursing mothers. Such outbreaks most often coincide with the appearance of a new strain of antibiotic-resistant staphylococcus. A contemporaneous example is MRSA, which has rapidly become the most commonly isolated staphylococcal species in some areas (Berens, 2010; Klevens, 2007). At Parkland Hospital from 2000 to 2004, Laibl and associates (2005) reported that a fourth of community-acquired MRSA isolates were from pregnant or postpartum women with mastitis. Hospital-acquired MRSA may cause mastitis when the newborn becomes colonized after contact with nursery personnel who are colonized (Centers for Disease Control and Prevention, 2006). Staford and colleagues (2008) found a higher incidence of recurrent abscess in those with MRSA-associated mastitis.
■ Management
Provided that appropriate therapy for mastitis is started before suppuration begins, the infection usually resolves within 48 hours. Many recommend that milk be expressed from the affected breast onto a swab and cultured before beginning therapy. Bacterial identification and antimicrobial sensitivities can provide information for a successful program of nosocomial infection surveillance (Lee, 2010).
The most effective treatment has not been clarified (Jahanar, 2013). Thus, the initial antimicrobial choice is influenced by current experience with staphylococcal infections at a given institution. Dicloxacillin, 500 mg orally four times daily, may be started empirically. Erythromycin is given to women who are penicillin sensitive. If the infection is caused by resistant, penicillinase-producing staphylococcus species or if resistant organisms are suspected while awaiting the culture results, then vancomycin, clindamycin, or trimethoprim-sulfamethoxazole is given (Shefeld, 2013). Although clinical response may be prompt, treatment is recommended for 10 days. Marshall and coworkers (1975) demonstrated the importance of continued breastfeeding. They reported that of 65 women with
mastitis, the only three who developed abscesses were among the 15 women who quit breastfeeding. Vigorous milk expression may be sufficient treatment alone (Tomsen, 1984). Sometimes the infant will not nurse on the inflamed breast. This probably is not related to any changes in the milk taste but is secondary to engorgement and edema, which can make the areola harder to grip. Pumping can alleviate this. When nursing bilaterally, it is best to begin suckling on the uninvolved breast. This allows let-down to commence before moving to the tender breast. In resource-poor countries, breastfeeding in women infected with the human immunodeficiency virus (HIV) is not contraindicated. However, in the setting of mastitis or breast abscess, it is recommended to stop feeding from the infected breast. This is because HIV RNA levels rise in affected breast milk. Tese levels return to baseline after symptoms resolve (Semrau, 2013).
■ Breast Abscess
In a population-based study of nearly 1.5 million Swedish women, the incidence of breast abscess was 0.1 percent (Kvist, 2005). An abscess should be suspected when defervescence does not follow within 48 to 72 hours of treatment or when a mass is palpable. Again, sonographic imaging is valuable. Breast abscesses can be large, and in one case report, 2 L of pus were released (Martic, 2012). Traditional therapy has been surgical drainage, which usually requires general anesthesia. The incision ideally is placed along Langer skin lines for a cosmetic result (Stehman, 1990). In early cases, a single incision over the most dependent portion of fuctuation is usually sufficient. Multiple abscesses require several incisions and disruption of loculations.
The resulting cavity is loosely packed with gauze, which should be replaced at the end of 24 hours by a smaller pack. More recently, sonographically guided needle aspiration using local analgesia has become favored (Patani, 2018). This has an 80- to 90-percent success rate (Geiss, 2014). In a randomized trial, Naeem and colleagues (2012) compared surgical drainage with aspiration. At 8 weeks, they found 93 percent undergoing aspiration were healed compared with 77 percent undergoing surgical drainage. Sonographic findings, initial choice of antimicrobials, and infecting organism do not predict aspiration failure (David, 2018).
Other etiologies should be considered in the setting of a nonhealing abscess. Rarely, granulomatous mastitis presents as puerperal mastitis (Ding, 2021 Freeman, 2017). Cancer or tuberculosis are other considerations (Wu, 2020).








Nhận xét
Đăng nhận xét