Chapter 7.2 Conventional Laparoscopy Myomectomy
GENERAL PRINCIPLES
Definition
■ Uterine leiomyomas are the most common gynecologic tumor, with an
incidence approximating 20% to 25% in women aged 18 to 65 years.1
Uterine myomas are discreet, sharply circumscribed masses, and
histologically appear as whorled bundles of smooth muscle. Most
commonly, leiomyomas grow within the uterine corpus, but may also occur
within the uterine ligaments, uterine cervix, or on other abdominal
structures. Individual myomas are believed to be monoclonal and result
from somatic mutations yielding dysregulation of genes involved in growth
regulation. Growth of uterine leiomyomas lead to uterine enlargement,
which may yield symptoms such as abnormal uterine bleeding,
dysmenorrhea, dyspareunia, subfertility, pelvic pressure, urinary frequency,
or defecatory dysfunction. Uterine myomectomy is the preferred surgical
therapy for management of symptomatic uterine myomas in women who
desire future pregnancy or uterine preservation. Laparoscopic myomectomy
is a feasible, minimally invasive procedure and has been demonstrated to
yield less pain, shorter hospital stays, improved cosmesis, less blood loss,
and faster recovery compared to laparotomic procedures, and similar
surgical outcomes with less overall cost compared to robot-assisted
procedures.
Differential Diagnosis
■ Adenomyosis
■ Congenital uterine anomaly
■ Endometrial polyp
■ Hematometra
■ Pregnancy
■ Uterine leiomyosarcoma
■ Uterine carcinosarcoma
■ Endometrial carcinoma
■ Metastatic disease
■ Tubo-ovarian neoplasm
Nonoperative Management■ The goal of nonoperative management for uterine leiomyomas is to
decrease symptomatology and improve the quality of life. To maximize
patient satisfaction and compliance, therapies must be convenient for the
patient and with minimal deleterious side effects. From a surgical
perspective, medical therapies for uterine leiomyomas are frequently
employed to raise preoperative hemoglobin levels or to reduce uterine
volume to optimize a patient prior to surgical intervention.
■ Observation, which entails no immediate intervention, is reasonable for
patients who experience minimal symptoms or who elect not to receive
treatment. Watchful waiting is a practical option to select women who are
approaching menopause as uterine leiomyomas often regress as circulating
levels of estradiol and progesterone naturally.
■ Steroid hormones are commonly employed to manage bothersome
symptoms attributable to uterine myomas. A common first-line therapy for
management of abnormal uterine bleeding and dysmenorrhea is
combination oral contraceptive pills (OCPs). Although the efficacy of
combination OCPs for the treatment of symptomatic fibroids is uncertain
and scientific evidence is scarce,2 oral contraceptives may sufficiently
reduce the bothersome symptoms attributable to leiomyoma uteri in certain
women. The levonorgestrel intrauterine system (LNG-IUS) is another option
for management of abnormal uterine bleeding and has been shown to
decrease menstrual blood loss, reduce uterine volume, and lead to
improvement in hemoglobin levels.3 Of note, the presence of submucous
myomas that significantly distort the uterine cavity is a relative
contraindication for LNG-IUS.
■ Administration of GnRH agonists results in downregulation of hypothalamic
GnRH receptors, ultimately inducing a reversible hypogonadal state by 2
weeks. The GnRH agonist, leuprolide acetate, is approved by the Food and
Drug Administration (FDA) to increase hemoglobin levels and decrease
myoma size prior to myomectomy. Women who receive leuprolide acetate
therapy typically develop amenorrhea within 3 months. The expected mean
reduction in uterine volume is 36% by 3 months and 45% by 6 months
following initiation therapy. Patients treated with GnRH agonists may
experience menopausal symptoms such as hot flushes, vaginal dryness,
mood changes, and a reversible decrease in bone density. Hormonal addback therapy may be initiated to minimize side effects from GnRH agonists.
Of note, preoperative treatment with leuprolide acetate may not improve
blood loss during myomectomy.
■ GnRH antagonists compete with endogenous GnRH for pituitary binding
sites and have the advantage of a comparatively rapid onset of clinical
effects without certain side effects observed with GnRH agonists. Evidencesuggests that a 31.3% reduction in uterine leiomyoma size can be achieved
by 14 days of treatment.4 GnRH antagonists currently available in the
United States require daily injections, which may be an obstacle for many
patients.
■ Mifepristone is a weak progesterone receptor agonist that has been
demonstrated to reduce heavy menstrual bleeding and improve myomaspecific quality of life. Treatment with mifepristone yields a reduction of
uterine volume by 26% to 74% in women with leiomyomas.5 At present,
mifepristone is not FDA-approved in the United States for the treatment of
uterine leiomyomas.
■ Ulipristal acetate (UPA) is an orally administered selective progesterone
receptor modulator that inhibits proliferation of leiomyoma cells, but not in
normal myometrial cells.6 UPA has been shown to significantly reduce
fibroid volume, decrease abdominal pressure, and decrease myoma-related
pain. UPA has stimulatory effects on the endometrium and its progesterone
antagonist action could result in an increased risk for endometrial
hyperplasia and endometrial carcinoma. However, studies have
demonstrated that the incidence of endometrial hyperplasia and malignancy
after treatment with UPA appears to be low. Pregnancies after UPA
administration have been reported without maternal complications related
to leiomyomas.
■ Magnetic resonance–guided focused ultrasound surgery (MRgFUS) is an
outpatient treatment option for uterine leiomyomas in premenopausal
women. MRgFUS is a noninvasive, thermoablative technique in which
waves of ultrasound energy converge on a small volume of tissue, which
leads to the thermal destruction, coagulative necrosis, and reduction of
leiomyoma of volume.7 Pregnancies have been described after MRgFUS
with no specific pattern of complications.
■ Uterine fibroid embolization (UFE) reduces uterine arterial blood flow and
results irreversible infarction of leiomyomas. The leiomyomas eventually
decrease in size and bothersome myoma-related symptoms improve. Most
commonly, the approach to percutaneous embolization is via the right or
left femoral artery under local anesthesia. UFE may reduce menstrual loss
by 85% and the mean dominant fibroid volume by 30% to 46%.8 The safety
of pregnancy after UFE has not been established to date; however,
pregnancies after UFE have been reported.9
IMAGING AND OTHER DIAGNOSTICS
■ Multiple imaging modalities are available for the evaluation of suspected
uterine myomas, each with relative strengths and weaknesses. In addition
to documenting the number and size of any myomas present, a primaryobjective of pelvic imaging prior to laparoscopic myomectomy is to rule
out other pathologies such as adenomyosis and gynecologic malignancy.
■ Pelvic ultrasound provides high-quality imaging of the uterus and adnexa
and is oftentimes the primary imaging modality for the evaluation of
patients with suspected uterine myomas (Fig. 7.2.1). Pelvic ultrasound is
widely available, with advantages that include relatively low cost, high
diagnostic accuracy, and lack of ionizing radiation. Ultrasound of the pelvis
is performed using both transabdominal and transvaginal techniques to
ensure the best-quality anatomic survey is obtained. It is important to note
that, although effective at evaluating total endometrial thickness,
transvaginal ultrasound has low sensitivity for detecting intracavitary
masses and determining the type of submucous myomas. Saline-infused
sonography (SIS) differs from nonenhanced transvaginal ultrasound in that
saline is employed to distend the uterine cavity, which serves as a contrast
medium and allows for detailed examination of the endometrium.
Figure 7.2.1. Pelvic ultrasound for evaluation fundal, subserosal myoma.
■ Magnetic resonance imaging (MRI) of the uterus is the preferred imaging
method for evaluating uterine myomas prior to laparoscopic myomectomy
(Fig. 7.2.2). MRI allows for efficient, comprehensive evaluation of the
uterus and clearly demonstrates the size and location of myomas present.
Compared to SIS, MRI is superior at accurately estimating the degree of
submucosal myoma ingrowth into the endometrial cavity, which is crucial
for preoperative planning, as a surgeon may choose to excise certain
submucous myomas in hysteroscopic fashion. MRI allows for assessment of
the junctional zone, which is important for identifying adenomyosis.Compared to computed tomography and pelvic ultrasound, MRI is superior
at differentiating benign uterine leiomyomas from uterine
leiomyosarcomas.
PREOPERATIVE PLANNING
■ Appropriate preoperative patient counseling is important to define a
patient’s goals from surgery and to establish reasonable expectations
following laparoscopic myomectomy. In general, the intent of uterine
myomectomy is to improve, not necessarily eliminate, bothersome
symptoms attributed to uterine myomas. The risks of laparoscopic
myomectomy should be emphasized, including perioperative blood loss
possibly necessitating transfusion, postoperative adhesion formation which
may yield pain or subfertility, and the rare need for hysterectomy if
unexpected pathology or uncontrollable bleeding is encountered.
Additionally, the patient should be informed of the possibilities of future
myoma recurrence and of intrapartum uterine rupture. The patient should
understand that any procedure that begins laparoscopically may require
laparotomy to complete.
Figure 7.2.2. Pelvic MRI for evaluation of fundal, subserosal myoma.
■ Favorable surgical outcomes require that medical comorbidities are
optimized prior to laparoscopic myomectomy. Commonly, patients with
abnormal uterine bleeding have iron deficiency anemia, which should be
corrected prior to surgery to minimize risk of perioperative transfusion andmaximize wound healing potential. Options for iron repletion include oral
iron therapy and intravenous iron infusions. Oftentimes, concomitant
medical therapy is required to reduce abnormal uterine bleeding in order to
achieve a net increase in hemoglobin. Pre-existing cardiopulmonary disease
may lead to difficulty with ventilation or tolerance of Trendelenburg
position and should be addressed prior to laparoscopic surgery. Poorly
controlled diabetes mellitus may lead to poor wound healing and increased
risk of perioperative infection.
SURGICAL MANAGEMENT
■ Laparoscopic myomectomy should be avoided in patients with suspected
gynecologic malignancy and contraindications to pneumoperitoneum or
Trendelenburg positioning. Other limitations to laparoscopic myomectomy
include the size and number of myomas to be excised and a surgeon’s
ability to efficiently perform laparoscopic suturing. In many instances, it is
more difficult to remove numerous small myomas as opposed to fewer,
larger myomas.
Positioning (Figs. 7.2.1 and 7.2.3)
■ The patient is placed in dorsal lithotomy position with their legs in stirrups
and their arms tucked in neutral positions at their sides. As Trendelenburg
positioning will eventually be necessary for the completion of the surgery,
it is prudent to take the opportunity to place the patient in Trendelenburg
position prior to surgical preparation to ensure that patient does not shift
on the operative table. Beanbag or foam egg crate mattress covers are
effective measures to ensure the patient’s position does not change during
Trendelenburg positioning. Following surgical preparation of the abdomen
and vagina, the patient is draped, a Foley catheter is introduced into the
bladder, and a uterine manipulator is placed. We prefer to use a uterine
manipulator through which fluid can be introduced into the uterine cavity
and allow performance of chromopertubation.
■ Placement of laparoscopic cannulas is dependent on the size and position of
the myomas. In general, we place laparoscopic cannulas at the umbilicus,
within the left and right epigastrum at the level of the umbilicus, and in the
left lower quadrant to facilitate laparoscopic suturing in an ipsilateral
approach from the patient’s left side (Fig. 7.2.3). As total uterine length
increases, port placement may need to shift cephalad, but the overall
geometry of the port placement need not change. The diameter of each port
depends on surgeon preference, myoma size, and quality of the
laparoscopic instruments.
Figure 7.2.3. Typical port configuration for laparoscopic myomectomy.Procedures and Techniques (Video 7.2)
Techniques to minimize perioperative blood loss
■ Laparoscopic myomectomy can be associated with significant intraoperative
blood loss, which may necessitate the perioperative transfusion of blood
products. The knowledge of the various agents and techniques that reduce
bleeding during laparoscopic myomectomy is essential to enhancing patient
safety and surgical outcomes. Several pharmacologic agents have been found to
reduce intraoperative blood loss during myomectomy. Preoperative
administration of uterotonics such as methylergonovine, misoprostol, and
prostaglandin E2 has been shown to decrease intraoperative blood loss by
evoking myometrial contractions.10 The intramyometrial injection of vasopressin
leads to reduction of intraoperative blood loss by evoking local vasoconstriction.
In our practice, 20 units of vasopressin are mixed with 100 mL of normal saline
and injected to form a wheal over the underlying leiomyoma. The half-life of
intramuscular vasopressin is 10 to 20 minutes and a surgeon should appreciate
that the safe maximal dosage of vasopressin has not been established. Care
should be taken to avoid intravascular injection, as there are rare cases in which
injection of vasopressin evoked cardiovascular collapse. Intravenous
administration of tranexamic acid (TXA) competitively inhibits the conversion of
plasminogen to plasmin, thereby minimizing intraoperative blood loss during
myomectomy by exerting an antifibrinolytic effect.11 Of note, the use of
intravenous oxytocin and locally injected bupivacaine with epinephrine have not
been demonstrated to decrease blood loss during myomectomy.12 Use of cell
salvage devices decreases the need for perioperative allogeneic blood
transfusion. Interventions that decrease blood flow to the uterus, such as uterine
artery embolization, application of a pericervical tourniquet, and temporary
clamping of the uterine artery, have been described with varying levels of
efficacy.
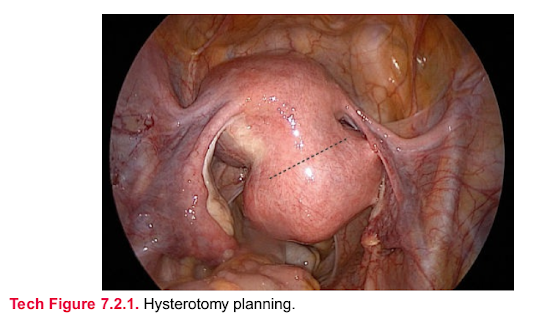
Hysterotomy
■ Hysterotomy planning is important. The planned incision must be of adequate
length to allow for efficient enucleation of the myoma, but without undue trauma to
healthy uterine tissue. The surgeon must ensure that vital structures such as the
fallopian tubes and ascending uterine vessels are not at risk for injury (Tech Fig.
7.2.1). Transverse uterine incision facilitates ipsilateral laparoscopic suturing.
However, some myomas in the inferior aspects of the uterus may require a
vertical or oblique incision to facilitate enucleation and hysterorrhaphy. Multiple
myomas can be enucleated through a single hysterotomy when possible.
Tech Figure 7.2.1. Hysterotomy planning.
■ Vasopressin (20 units mixed with 100 cc normal saline) is injected into the
myometrium surrounding the myoma (Tech Fig. 7.2.2). Depending on the depth of
the myoma, tissue blanching and wheal formation may be observed. Hysterotomy
is performed using either a monopolar radiofrequency (RF) instrument or an
ultrasonic scalpel. For both classes of instruments, the surgical goal is to rapidly
incise tissue with the aim of minimizing unintended thermal injury. When using a
monopolar instrument, the electrosurgical unit is set to output a low-voltage,
continuous (“cut”) waveform. The uterine tissue is incised by linear vaporization, a
nontouch technique in which RF energy arcs from the tip of the active electrode
to the target tissue, leading to rapid tissue division with minimal thermal spread.
When using an ultrasonic scalpel, the device’s maximum blade excursion setting
should be used and care taken to minimize dwell time at the surgical site (Tech
Fig. 7.2.3). Incidental bleeding from the serosa and myometrium can be controlled
by judicious use of RF or ultrasonic energy. In general, excessive use of energy
devices to control myometrial bleeding is ill advised due to the possibility of
tissue necrosis and poor wound healing. If significant bleeding is encountered,
hemostasis may be achieved by suture ligation or pressure application.
Hysterotomy proceeds until the capsule of the myoma is observed (Tech Fig.
7.2.4).
Tech Figure 7.2.2. Injection of dilute vasopressin solution into myometrium surrounding
the myoma.
Tech Figure 7.2.3. Hysterotomy performed with ultrasonic scalpel.
Tech Figure 7.2.4. Exposure of the myoma capsule following hysterotomy.
Enucleation of myoma
■ The capsule of the myoma is incised (Tech Fig. 7.2.5) and dissection proceeds
between the inner capsule and myoma edge (Tech Fig. 7.2.6). If developing the
appropriate plane proves difficult, one can make a shallow incision in the myoma
itself, which may help distinguish myoma from capsule. Application of traction on
the myoma and countertraction on the capsule provides surgical exposure and
aids in the enucleation of the myoma. Once excised, the myoma is placed in the
posterior cul-de-sac for later retrieval (Tech Fig. 7.2.7). It is important for the
surgical team to keep an accurate count of the number of myomas excised to
ensure all specimens are extracted from the abdomen at the end of the
procedure. The myoma capsule need not be excised. Additionally, incising the
endometrium should be avoided if possible. However, entering the cavity is
unavoidable in the case of certain submucous myomas. If breach of the
endometrial cavity is in question, a solution of methylene blue may be introduced
into the endometrium through the uterine manipulator and any defects identified.
Tech Figure 7.2.5. Exposure of myoma following incision of myoma capsule.
Tech Figure 7.2.6. Enucleation of myoma by dividing tissue investments between
myoma and myoma capsule.
Tech Figure 7.2.7. Stowage of myoma in posterior cul-de-sac for later retrieval.
Hysterorrhaphy
■ Using laparoscopic suturing techniques, the myometrium is approximated in
multiple layers using 0 or 2-0 gauge, delayed absorbable suture in running or
figure-of-eight fashion. Studies have established that the use of barbed suture is
safe and effective for hysterotomy closure, with overall less time required for
hysterorrhaphy (Tech Fig. 7.2.8).13 Care must be taken to effectively approximate
tissue and achieve hemostasis, without undue tension being placed on the
myometrium which may lead to tissue strangulation. The serosa is approximated
using either 2-0 delayed absorbable conventional or barbed suture in simple
running or baseball stitch fashion. If the uterine cavity is entered during
enucleation of a submucous myoma, the endometrium should be approximated
with 3-0 delayed-absorbable, monofilament suture in simple running fashion.
Chromopertubation, if indicated, may be performed after hysterorrhaphy
completion (Tech Fig. 7.2.9).
Tech Figure 7.2.8. Hysterorrhaphy using delayed-absorbable barbed suture.
Tech Figure 7.2.9. Completion of hysterorrhaphy.
Myoma extraction
■ The FDA has discouraged tissue extraction by laparoscopic power morcellation
due to the risk of spreading unsuspected cancer during the process. Multiple
groups are evaluating the potential utility of contained electromechanical
morcellation, but data are sparse at present. However, other methods are
available for extracting myomas following laparoscopic myomectomy.
■ Minilaparotomy is efficient and effective for tissue extraction in most cases.
Following hysterorrhaphy, the myomas are placed securely in a laparoscopic
specimen bag (Tech Fig. 7.2.10). Depending on the patient’s anatomy, myoma
size, and prior surgical incisions, minilaparotomy is performed either through a
transverse Pfannenstiel incision or an extended umbilical incision. Thelaparoscopic bag is brought through the incision and the myomas are removed
(Tech Fig. 7.2.11). Again, it is important to confirm the number of myomas
removed matches the number of myomas enucleated. For larger myomas,
contained morcellation with a scalpel is feasible and can be facilitated by
placement of a self-retaining wound retractor.
■ Posterior colpotomy is a reasonable approach for extracting moderate-sized
myomas in appropriately selected patients. Again, myomas are carefully placed
within a laparoscopic retrieval bag. A posterior colpotomy is made from the
medial aspect of one uterosacral ligament to the medial aspect of the
contralateral uterosacral ligament using either a monopolar RF or an ultrasonic
device. Posterior colpotomy is facilitated by elevating the posterior vaginal fornix
cephalad, either by employing a uterine manipulator with a pericervical cup or an
assistant with a Breisky retractor placed within the posterior fornix (Tech Fig.
7.2.12). Ring forceps are introduced through the colpotomy and the string of the
laparoscopic retrieval bag grasped (Tech Fig. 7.2.13). The myomas counted to
confirm all specimens are extracted. The posterior colpotomy is repaired using
2-0 delayed-absorbable suture is running or figure-of-eight fashion using either
laparoscopic or transvaginal suturing technique.
Tech Figure 7.2.10. Collection of myoma in laparoscopic retrieval bag in anticipation of
contained transabdominal tissue extraction.
Tech Figure 7.2.11. Extraction of myoma through transverse mini-Pfannenstiel incision.
Tech Figure 7.2.12. Posterior colpotomy along pericervical cup.
Tech Figure 7.2.13. Extraction of myoma tissue through posterior colpotomy.
Adhesion prevention
■ Following tissue extraction, the pelvis is inspected laparoscopically. Meticulous
hemostasis is achieved by holding pressure, use of RF energy, suture ligation, or
hemostatic agent. An adhesion barrier such as oxidized regenerated cellulose is
placed over each hysterotomy to decrease incidence of postoperative pelvic
adhesion formation (Tech Fig. 7.2.14).
Tech Figure 7.2.14. Oxidized regenerated cellulose placed over hysterorrhaphy site for
adhesion prevention.PEARLS AND PITFALLS
CASE SELECTION
Laparoscopic myomectomy is associated with a learning curve and perioperative
outcomes are dependent on multiple factors. A surgeon should individualize their
surgical approach for myomectomy based on factors including myoma size, number
of myomas present, and their personal experience and skill level.
BARBED SUTURE
The use of barbed suture facilitates efficient hysterotomy closure and can reduce the
length of surgery in some cases.
UNDETECTED UTERINE PATHOLOGY
MRI for preoperative evaluation of uterine fibroids is most likely to detect the
presence of pathologies such as adenomyosis and sarcoma compared to other
imaging modalities and is the preferred method for evaluating a patient considering
surgical myomectomy.
POSTOPERATIVE CARE
■ Patients who undergo laparoscopic myomectomy can typically be
discharged home the same day. Common clinical scenarios that necessitate
inpatient management include pain not controlled by oral pain medications,
persistent nausea or vomiting, or for perioperative management of
comorbidities. Patients are prescribed a narcotic pain medication, stool
softener, antiemetic, and nonsteroidal anti-inflammatory medication.
Patients are instructed to ambulate as tolerated, but refrain from strenuous
activity for 2 weeks. Patients with tissue extraction via posterior colpotomy
are instructed to refrain from vaginal intercourse for 6 weeks. Patients are
counseled to expect mild uterine cramping and light bleeding per vagina.
They are instructed to seek medical attention should they experience
intractable pain, unrelenting nausea, fever greater than 38.3°C, excessive
vaginal bleeding, lightheadedness, shortness of breath, or syncope. Patients
are typically examined at 6 weeks following surgery. We advise patients to
not conceive for 3 to 6 months following laparoscopic myomectomy.
OUTCOMES
■ Studies report a steady increase in the cumulative recurrence rate of uterine
myomas following laparoscopic myomectomy, specifically 11%, 36%, 53%,
and 84% at 1, 3, 5, and 8 years, respectively.14 However, recurrence doesnot always require further treatment. A study of 114 patients noted that at
a 27-month interval after laparoscopic myomectomy, 33% of patients had
recurrent leiomyomas; however, only 37% of the patients required
additional surgical management.15
COMPLICATIONS
■ Laparoscopic myomectomy has clear advantages over abdominal
myomectomy by avoiding risks associated with laparotomy. A metaanalysis found that mean operative times were 13 minutes longer in the
laparoscopic myomectomy group,16 but the lengths of hospital stay and
time required for recovery are significantly lower in laparoscopic
myomectomies compared to laparotomic myomectomies. A multicenter
study including 2,050 patients reported the total complication rate of
laparoscopic myomectomy to be 11.1% with minor complications
accounting for 9.1% and major complications for 2.02%.17 The most serious
reported complications were perioperative hemorrhage in 0.68% and
postoperative hematomas in 0.48%.17 A known complication of
myomectomy is intra- and postoperative bleeding, and blood loss has been
shown to be significantly less in laparoscopic myomectomy compared to
abdominal myomectomy.18
■ The formation of adhesions is a frequently encountered complication
following myomectomy. Studies, in which a second-look laparoscopy was
performed following laparoscopic myomectomy, have reported intraabdominal adhesions to be present in up to 66% of women.19 Adhesion
prevention at time of myomectomy should be considered a priority and
multiple agents have been found to reduce the rate of adhesion formation,
such as oxidized regenerated cellulose, which was noted to significantly
decrease the rate of adhesion formation to 12% at the time of second-look
laparoscopy, compared to 60% without use of adhesion barrier.20
■ Intrapartum uterine rupture is a rare and potentially life-threatening
pregnancy complication after myomectomy and manifests with symptoms
of vaginal bleeding, fetal distress on cardiotocographs, increased uterine
contractions, pain, and loss of fetal station. Breach of the endometrium
during myomectomy may increase this risk and, as such, women may be
offered elective cesarean delivery of a subsequent pregnancy to minimize
this risk. Expert opinion suggests that a multilayered uterine closure and
avoidance of excessive use of RF energy on the myometrium are
appropriate measures to reduce the risk of subsequent uterine rupture after
myomectomy.21 A study evaluated 359 women after laparoscopic
myomectomy and 72 women became pregnant resulting in 76 pregnancies
in which no case of uterine rupture or dehiscence occurred.22 The authorssupposed that their favorable results may be explained by the meticulous
hemostasis that was obtained and by a layered closure of hysterotomies, as
well the avoidance of excessive cautery. The true incidence of uterine
rupture after myomectomy, whether laparoscopic or abdominal, is
unknown. No data suggest that one suturing technique or material is
superior in minimizing this risk of uterine rupture.23
■ A significant complication associated with minimally invasive myomectomy
is dissemination of uterine tissue during enucleation and tissue extraction.
Iatrogenic, parasitic myoma formation is a rare complication following
myomectomy and has been observed in patients who underwent manual or
electromechanical morcellation techniques. The enucleation and extraction
of an unsuspected uterine sarcoma may lead to intra-abdominal spread of
cancerous tissue and worsen a patient’s long-term survival. Presently, the
FDA warns that an estimated 1 in 350 women undergoing hysterectomy or
myomectomy for treatment of uterine myomas are found to have an
unsuspected uterine sarcoma. Patients should
















Nhận xét
Đăng nhận xét