Female reproductive system
BS. Nguyễn Hồng Anh
UTERUS
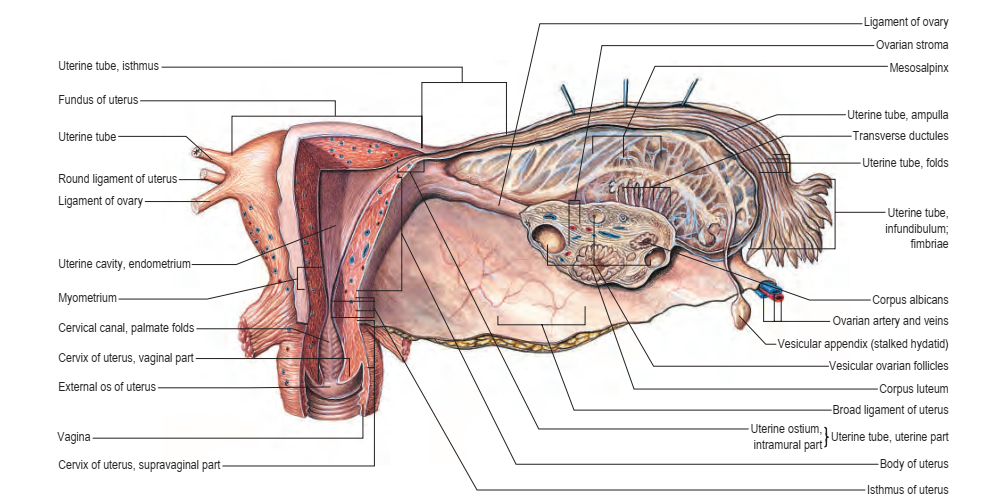
The uterus is a thick-walled, muscular organ situated in the pelvis between the urinary bladder and the rectum (Figs 77.10–77.12). It lies posterior to the bladder and uterovesical space, and anterior to the rectum and recto-uterine pouch; it is mobile, which means that its position varies with distension of the bladder and rectum. The broad ligaments are lateral. The uterus is divided structurally and functionally into two main regions: the muscular body of the uterus (corpus uteri) forms the upper two-thirds, and the fibrous cervix (cervix uteri) forms the lower third In the adult nulliparous state, the cervix usually tilts forwards relative to the axis of the vagina (anteversion), and the body of the uterus tilts forwards relative to the cervix (anteflexion) (see Fig. 77.12). In 10–15% of women, the whole uterus leans backwards at an angle to the vagina and is said to be retroverted. A uterus that angles backwards on the cervix is described as retroflexed.
Body
The body of the uterus is pear-shaped and extends from the fundus superiorly to the cervix inferiorly. The uterine tubes enter the uterus on both sides at the uterine cornua. The round and ovarian ligaments are inferoanterior and inferoposterior, respectively, to each cornu. The dome-like fundus is superior to the entry points of the uterine tubes and covered by peritoneum that is continuous with that of neighbouring surfaces. The fundus is in contact with coils of small intestine and, occasionally, by distended sigmoid colon. The lateral margins of the body are convex; on each side, their peritoneum is reflected laterally to form the broad ligament, which extends as a flat sheet to the pelvic wall
Fig. 77.11 A, Anatomical relations of the female genital tract, bladder and rectum. B, Pelvic peritoneal reflections, showing the broad ligament and its contents. (A, With permission from Drake RL, Vogl AW, Mitchell A, Tibbitts R, Richardson P (eds), Gray’s Atlas of Anatomy, Elsevier, Churchill Livingstone. Copyright 2008. B, With permission from Drake RL, Vogl AW, Mitchell A (eds), Gray’s Anatomy for Students, 2nd ed, Elsevier, Churchill Livingstone. Copyright 2010.) (see Figs 77.11B, 77.15). The anterior surface of the uterine body is covered by peritoneum reflected on to the bladder at the uterovesical fold (see Fig. 77.14). This normally occurs at the level of the internal os, the most inferior margin of the body of the uterus. The vesicouterine pouch, between the bladder and uterus, is obliterated when the bladder is distended, but may be occupied by small intestine when the bladder is empty. The posterior surface of the uterus is convex transversely. Its peritoneal covering continues down to the cervix and upper vagina, and is then reflected back to the rectum along the surface of the recto-uterine pouch, which lies posterior to the uterus (see Fig. 77.16).
The sigmoid colon, and occasionally the terminal ileum, lie posterior to the uterus. The cavity of the uterine body usually measures 6 cm from the external os of the cervix to the wall of the fundus and is flat in its anteroposterior plane (Salardi et al 1985). In coronal section, it is triangular, broad above where the two uterine tubes join the uterus, and narrow below at the internal os of the cervix. There is no change in the size of the uterus until approximately 7 years of age, when there is greater enlargement of the body of the uterus than the cervix.
Developmental anomalies of the uterus There may be failure in fusion of the paramesonephric (Müllerian) ducts, which results in a uterus that is not pear-shaped. There may only be a septum (septate uterus) or partial clefting of the uterus (bicornuate uterus); the most extreme example is a septate vagina, two cervices and two discrete uteri, each with one uterine tube (uterus didelphys) (Minto et al 2001) (see Fig. 72.15).
Cervix
The adult, non-pregnant cervix is narrower and more cylindrical than the body of the uterus and is typically 2.5 cm long. The upper end communicates with the uterine body via the internal os, and the lower end opens into the vagina at the external os (see Fig. 77.6). In nulliparous women, the external os is usually a circular aperture, whereas, after childbirth, it is a transverse slit. Two longitudinal ridges, one each on its anterior and posterior walls, give off small, oblique, palmate folds that ascend laterally like the branches of a tree (arbor vitae uteri); the folds on opposing walls interdigitate to close the canal. The narrower isthmus forms the upper third of the cervix. Although unaffected in the first month of pregnancy, it is gradually taken up into the uterine body during the second month to form the ‘lower uterine segment’ (see below). In nonpregnant women, the isthmus undergoes menstrual changes, although these are less pronounced than those occurring in the uterine body. The external end of the cervix enters the upper end of the vagina, thereby dividing the cervix into supravaginal and vaginal parts. The supravaginal part is separated anteriorly from the bladder by cellular connective tissue: the parametrium, which also passes to the sides of the cervix and laterally between the two layers of the broad ligaments.
Peritoneal folds and ligaments of the pelvis
The uterus is connected to a number of ‘ligaments’. Some are true ligaments, in that they have a fibrous composition and provide support to the uterus; some provide no support to the uterus; and others are simply folds of peritoneum.
Peritoneal folds
The parietal peritoneum is reflected over the upper genital tract to produce anterior (uterovesical), posterior (rectovaginal) and lateral peritoneal folds. The lateral folds are commonly called the broad ligaments (Fig. 77.13)
uterovesical and rectovaginal folds
The anterior, or uterovesical, fold consists of peritoneum reflected on
to the bladder from the uterus at the junction of its cervix and body
(Fig. 77.14). The posterior or rectovaginal fold extends lower than the
anterior fold and consists of peritoneum reflected from the posterior
vaginal fornix on to the front of the rectum, thereby creating the deep
recto-uterine pouch (pouch of Douglas). The recto-uterine pouch is
bounded anteriorly by the uterus, supravaginal cervix and posterior
vaginal fornix; posteriorly, by the rectum; and laterally, by the uterosacral ligaments.
Broad ligament
The lateral folds, or broad ligaments, extend on each side from the
uterus to the lateral pelvic walls, where they become continuous with
the peritoneum covering those walls (Figs 77.15, 77.17–77.18). The
upper border is free and the lower border is continuous with the peritoneum over the bladder, rectum and side wall of the pelvis. The borders
are continuous with each other at the free edge via the uterine fundus,
and diverge below near the superior surfaces of levatores ani. A uterine
tube lies in the upper free border on either side. The broad ligament is
divided into an upper mesosalpinx, a posterior mesovarium and an
inferior mesometrium.
Mesosalpinx
The mesosalpinx is attached above to the uterine tube and posteroinferiorly to the mesovarium (see Fig. 77.17). Superior and laterally, it is
attached to the suspensory ligament of the ovary; medially, it is attached
to the ovarian ligament. The fimbria of the tubal infundibulum projects
from its free lateral end. Between the ovary and uterine tube, the mesosalpinx contains vascular anastomoses between the uterine and
ovarian vessels, the epoophoron and the paroophoron. The mesovarium projects from the posterior aspect of the broad ligament, of
which it is the smaller part. It is attached to the hilum of the ovary and
carries vessels and nerves to the ovary.
Mesometrium
The mesometrium is the largest part of the broad ligament, and extends
from the pelvic floor to the ovarian ligament and uterine body. The
uterine artery passes between its two peritoneal layers typically 1.5 cm
lateral to the cervix; it crosses the ureter shortly after its origin from the
internal iliac artery and gives off a branch that passes superiorly to the
uterine tube, where it anastomoses with the ovarian artery (Fig. 77.19).
Between the pyramid formed by the infundibulum of the tube, the
Fig. 77.17 Pelvic peritoneal
reflections, demonstrating the
broad ligament and its contents.
(With permission from Drake RL,
Vogl AW, Mitchell A (eds),
Gray’s Anatomy for Students,
2nd ed, Elsevier, Churchill
Livingstone. Copyright 2010.)
Fig. 77.18 The ovaries and broad ligament: superior view with the uterus lifted away from the bladder. (With permission from Waschke J, Paulsen F (eds), Sobotta Atlas of Human Anatomy, 15th ed, Elsevier, Urban & Fischer. Copyright 2013.) upper pole of the ovary, and the lateral pelvic wall, the mesometrium contains the ovarian vessels and nerves lying within the fibrous suspensory ligament of the ovary (infundibulopelvic ligament). This ligament continues laterally over the external iliac vessels as a distinct fold. The mesometrium also encloses the proximal part of the round ligament of the uterus, as well as smooth muscle and loose connective tissue.
Ligaments of the pelvis
The ligaments of the pelvis consist of the round, uterosacral, transverse cervical and pubocervical ligaments.
Round ligament
Each round ligament is a narrow smooth muscle band 10–12 cm long, which extends from the lateral cornu of the uterus through the broad ligament to enter the deep inguinal ring lateral to the inferior epigastric artery (see Figs 77.13, 77.15, 77.18). Although conventionally described as ending in the labium majus, a cadaveric dissection study found that, in girls, the round ligament ended just outside the external ring, with neither attachment nor extension to the caudal labium (the homologue of the hemiscrotum) (Attah and Hutson 1991). Near the uterus, the round ligament contains a considerable amount of smooth muscle but this gradually diminishes and the terminal portion is purely fibrous.
The round ligament also contains striated muscle, blood vessels, nerves and lymphatics. The latter drain the uterine region around the entry of the uterine tube to the superficial inguinal lymph nodes.
In the fetus, a projection of peritoneum (processus vaginalis) is carried with the round ligament for a short distance into the inguinal canal. This is generally obliterated in adults, although it is sometimes patent even in old age. A patent processus vaginalis in the inguinal canal in females is often referred to as the canal of Nuck; it may be asymptomatic or it may give rise to an inguinal hernia or hydrocele of the canal of Nuck. In the canal, the ligament receives the same coverings as the spermatic cord, although they are thinner and blend with the ligament itself, which may not reach the mons pubis. The round and ovarian ligaments both develop from the gubernaculum and are continuous uterosacral, transverse cervical and pubocervical ligaments
The uterosacral, transverse cervical and pubocervical ligaments are condensations of the visceral or endopelvic connective tissue that connect the pelvic viscera to the side wall of the pelvis; they radiate like the spokes of a wheel around the hub of the cervix, providing it with considerable support (see Figs 77.13–16) (Delancey 2011). The connective tissue lateral to the uterus and the cervix – the parametrium – continues down along the vagina as the paracolpium. The uterosacral ligaments contain fibrous tissue and smooth muscle. They pass back from the cervix and uterine body on both sides of the rectum, and are attached to the anterior aspect of the sacrum. They can be palpated laterally on rectal examination and can be felt as thick bands of tissue passing downwards on both sides of the posterior fornix on vaginal examination. The transverse cervical ligaments (cardinal ligaments, ligaments of Mackenrodt) (Fig. 77.20) extend from the side of the cervix and lateral fornix of the vagina, and are attached extensively on the pelvic wall.
The lower parts of the ureters and pelvic blood vessels traverse the transverse cervical ligaments. Fibres of the pubocervical ligament pass forwards from the anterior aspect of the cervix and upper vagina to diverge around the urethra, and are attached to the posterior aspect of the pubic bones.
Fig. 77.19 The broad ligament (left), and blood supply to the uterus and ovaries (right). (With permission from Drake RL, Vogl AW, Mitchell A, Tibbitts R, Richardson P (eds), Gray’s Atlas of Anatomy, Elsevier, Churchill Livingstone. Copyright 2008.)
The transverse cervical and uterosacral ligaments are almost vertically orientated in the standing position and maintain the near horizontal axis of the upper vagina. The uterus and vagina are supported by the close interaction of the uterosacral and transverse cervical ligaments with the muscles of the pelvic floor, including the levatores ani and coccygei, the perineal membrane and the perineal body. The support of the pelvic floor has been reviewed in detail by Delancey (2011) (see also Ch. 73).
Vascular supply and lymphatic drainage
Arteries
The arterial supply to the uterus comes from the uterine artery (see Fig. 77.19; Fig. 77.21), which arises as a branch of the anterior division of the internal iliac artery. From its origin, the uterine artery crosses the ureter anteriorly in the broad ligament before branching as it reaches the uterus at the level of the cervico-uterine junction (see Fig. 74.21B).
One major branch ascends the uterus tortuously within the broad ligament until it reaches the region of the ovarian hilum, where it anastomoses with branches of the ovarian artery. Another branch descends to supply the cervix and anastomoses with branches of the vaginal artery to form two median longitudinal vessels: the azygos arteries of the vagina, which descend anterior and posterior to the vagina. Although there are anastomoses with the ovarian and vaginal arteries, the dominance of the uterine artery is indicated by its marked hypertrophy during pregnancy.
The tortuosity of the vessels as they ascend in the broad ligaments is repeated in their branches within the uterine wall. Each uterine artery gives off numerous branches. These enter the uterine wall, divide and run circumferentially as groups of anterior and posterior arcuate arteries. They ramify and narrow as they approach the anterior and posterior midline so that no large vessels are present in these regions. However, the left and right arterial trees anastomose across the midline and unilateral ligation can be performed without serious effects. Terminal branches in the uterine muscle are tortuous and are called helicine arterioles. They provide a series of dense capillary plexuses in the myometrium and endometrium. From the arcuate arteries, many helical arteriolar rami pass into the endometrium. Their detailed appearance changes during the menstrual cycle. In the proliferative phase, helical arterioles are less prominent, whereas they grow in length and calibre, becoming even more tortuous in the secretory phase.
Fig. 77.20 The supporting ligaments of the pelvis, showing the transverse cervical ligaments.
Veins
The uterine veins extend laterally in the broad ligaments, running adjacent to the arteries and passing over the ureters. They drain into the internal iliac veins (see Fig. 77.3). The uterine venous plexus anastomoses with the vaginal and ovarian venous plexuses.
Lymphatic drainage
Uterine lymphatics exist in the superficial (subperitoneal) and deep parts of the uterine wall. Collecting vessels from the body of the uterus and cervix pass laterally in the parametrium to three main groups of lymph nodes: the external and internal iliac and the obturator nodes (see Table 77.1). The external and internal iliac nodes surround their
corresponding arteries. The obturator nodes lie in the obturator fossa
between the external and internal iliac vessels; the obturator nerve
passes through the lower part of this group of lymph nodes.
Lymph vessels from the fundus of the uterus and the uterine
tubes may accompany the lymph drainage of the ovaries to para-aortic
nodes (see Fig. 77.3B). The region surrounding the isthmus of the
uterine tube may drain along the round ligament to the superficial
inguinal nodes.
Innervation
The nerve supply to the uterus is predominantly from the inferior hypogastric plexus (see Fig. 77.5) (Shoja et al 2013). Some branches ascend with,
or near, the uterine arteries in the broad ligament. They supply the uterine
body and tubes, and connect with tubal nerves from the inferior hypogastric plexus and with the ovarian plexus. The uterine nerves terminate in
the myometrium and endometrium, and usually accompany the vessels
(see Table 77.2). Nerves to the cervix form a plexus that contains small
paracervical ganglia. Sometimes, one ganglion is larger and is termed the
uterine cervical ganglion. Branches may pass directly to the cervix uteri or
may be distributed along the vaginal arteries.
Efferent preganglionic sympathetic fibres are derived from neurones
in the last thoracic and first lumbar spinal segments; the sites where
they synapse on their postganglionic neurones are unknown but are
presumably in the superior and/or inferior hypogastric plexuses (Lee
et al 1973). Preganglionic parasympathetic fibres arise from neurones
in the second to fourth sacral spinal segments and relay in the paracervical ganglia. Sympathetic activity may produce uterine contraction and
vasoconstriction, and parasympathetic activity may produce uterine
inhibition and vasodilation, but these activities are complicated by
hormonal control of uterine functions.
Microstructure
Body of the uterus
The uterus is composed of three main layers. From its lumen outwards,
these are the endometrium (mucosa), myometrium (smooth muscle
layer) and serosa (or adventitia) (Fig. 77.22).
Fig. 77.21 A, The normal arterial supply to the uterus and ovary. B–D, Variations in arterial blood supply to the ovary. E–F, Variations in arterial blood
supply to the fundus of the uterus. (A, With permission from Waschke J, Paulsen F (eds), Sobotta Atlas of Human Anatomy, 15th ed, Elsevier, Urban &
Fischer. Copyright 2013.)
Fig. 77.22 The uterus, uterine tubes and ovaries. (With permission from Waschke J, Paulsen F (eds), Sobotta Atlas of Human Anatomy, 15th ed, Elsevier,
Urban & Fischer. Copyright 2013.)
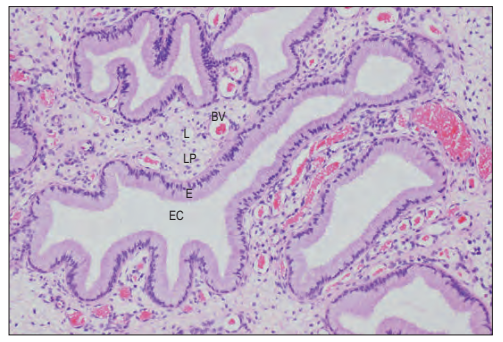
Fig. 77.23 The endocervical glands. These are are deep infoldings of the
lining of the endocervical canal (EC). The epithelium (E) is simple
columnar and mucus-secreting. The underlying lamina propria (LP) is
richly supplied with blood vessels (BV) and lymphatics (L). (Courtesy of Mr
Peter Helliwell and the late Dr Joseph Mathew, Department of
Histopathology, Royal Cornwall Hospitals Trust, UK.)
Fig. 77.24 The transformation zone of the uterine cervix. The singlelayered columnar epithelium lining the endocervical canal (EC) and its
endocervical glands (EG) changes abruptly (arrow) to the stratified
squamous non-keratinizing epithelium of the external os and ectocervix
(below arrow).
Endometrium
The endometrium is formed by a layer of connective tissue, the endometrial stroma, which supports a single-layered columnar epithelium.
Before puberty, the epithelium is ciliated and cuboidal. It contains
glands that are composed largely of columnar cells secreting glycoproteins and glycogen. After puberty, the structure of the endometrium
varies with the stage of the menstrual cycle (see below). The glands are
tubular, run perpendicular to the luminal surface and penetrate up to
the myometrial layer. The stroma consists of a highly cellular connective
tissue between the endometrial glands, and contains blood and lymphatic vessels.
myometrium
The myometrium is composed of smooth muscle and loose connective
tissue, and contains blood vessels, lymphatic vessels and nerves. It is
dense and thick at the uterine midlevel and fundus but thin at the tubal
orifices. The body of the uterus has four muscular layers. The submucosal (innermost) layer is composed of longitudinal and some oblique
smooth muscle fibres. Where the lumen of the uterine tube passes
through the uterine wall, this layer forms a circular muscle coat. The
vascular layer is external to the submucosal layer and is rich in blood
vessels, as well as longitudinal muscle; it is succeeded by a layer of
predominantly circular muscle, the supravascular layer. The outer, thin,
longitudinal muscle layer, the subserosal layer, lies adjacent to the
serosa.
The muscular fibres of the outer two layers converge at the lateral
angles of the uterus and continue into the uterine tubes. Some fibres
enter the broad ligaments as the round and ovarian ligaments; others
turn back into the uterosacral ligaments. At the junction between the
body and the cervix, the smooth muscle merges with dense, irregular
connective tissue containing both collagen and elastin, and forms the
majority of the cervical wall. Bilateral longitudinal fibres extend in the
lateral submucosal layer from the fundal angle to the cervix. Their
muscle fibres differ structurally from those of typical myometrium, and
they may provide fast-conducting pathways that coordinate the contractile activities of the uterine wall.
serosa
The uterine body is covered by peritoneal serosa, which continues
downwards posteriorly to cover the supravaginal cervix. The anterior
cervix and the lateral surfaces of the uterine body and cervix are not
covered by peritoneum.
Cervix uteri
The cervix consists of fibroelastic connective tissue and contains relatively little (10%) smooth muscle. The elastin component of the cervical
stroma is essential to the stretching capacity of the cervix during childbirth. The cervical canal is lined by a deeply folded mucosa with a
surface epithelium of columnar mucous cells (Fig. 77.23). There are
branched tubular glands present within the mucosa, which are lined by
a similar secretory epithelium. The glands extend obliquely upwards
and outwards from the canal. They secrete clear, alkaline mucus, which
is relatively viscous except at the midpoint of the menstrual cycle, when
it becomes more copious and less viscous to encourage the passage of
sperm. At the vaginal end of the cervix, the aperture of a gland may
block and it then fills with mucus to form a Nabothian follicle (or cyst).
None of the mucosa is shed during menstruation and so, unlike the
body of the uterus, it is not divided into functional and basal layers,
and lacks spiral arteries. The surface of the intravaginal part of the cervix
(ectocervix) is covered by non-keratinizing stratified squamous epithelium, which contains glycogen.
The squamocolumnar junction, where the columnar secretory epithelium of the endocervical canal meets the stratified squamous covering of the ectocervix, is located at the external os before puberty. As
oestrogen levels rise during puberty, the cervical os opens, exposing the
endocervical columnar epithelium on to the ectocervix. This area of
columnar cells on the ectocervix forms an area that is red and raw in
appearance, called an ectropion (cervical erosion). It is then exposed to
the acidic environment of the vagina and, through a process of squamous metaplasia (p. 40), transforms into stratified squamous epithelium. This area is thus known as the ‘transformation zone’ (Fig. 77.24).
Other hyperoestrogenic states, such as pregnancy and the use of oral
contraceptive pills, can also result in an ectropion. This area is the most
usual site of cervical intraepithelial neoplasia (CIN), which may
progress to malignancy. In postmenopausal women, the squamocolumnar junction recedes into the endocervical canal.
Magnetic resonance imaging of the uterus
and vagina
On T2-weighted magnetic resonance imaging (MRI), the uterus displays
a zonal anatomy, with three distinct zones: the endometrium, junctional zone and myometrium (Fig. 77.25) (Minto et al 2011). The
endometrium and uterine cavity appear as a high-signal stripe; the
thickness varies with the stage of the menstrual cycle. In the early proliferative phase, it measures up to 5 mm, and widens to up to 1 cm in
the mid-secretory phase. A band of low signal, the junctional zone,
borders the endometrium. It represents the inner myometrium and is
of constant thickness and signal throughout the menstrual cycle; it
usually measures 5 mm. The outer myometrium is of medium-signal
intensity in the proliferative phase, and of high-signal intensity in the
mid-secretory phase as a result of the increased vascularity and prominence of the arcuate vessels.
In prepubertal females, the uterus is smaller (only 4 cm in length),
and on T2-weighted images, the endometrium is minimal or absent,
and the junctional zone is indistinct. In postmenopausal women, the
corpus decreases in size and the zonal anatomy is indistinct.
On T2-weighted MRI, the cervix has an inner, low-signal stroma
continuous with the junctional zone of the uterus. Often, this is surrounded by an outer zone of intermediate signal intensity, which is
continuous with the outer myometrium. The appearances do not
change with the menstrual cycle or oral contraceptive pill use. The
central stripe is very high-signal and is a consequence of the secretions
produced by the endocervical glands.
The vagina is best seen on T2-weighted MRI as a thin layer of highsignal intensity of the mucosa and an outer, low-signal layer of the
submucosa and muscularis (see Fig. 77.25). MRI accurately demonstrates congenital and acquired Müllerian anomalies, including uterine
and vaginal aplasia, duplication and septa (Grant et al 2010). Vaginal
and perivaginal cysts are well visualized with T2-weighted MRI.
UTERINE (FALLOPIAN) TUBES
The uterine tubes are attached to the upper part of the body of the
uterus, and their ostia open into the uterine cavity (Figs 77.26, 77.27,
77.28). The medial opening of the tube (the uterine os) is located at
the superior angle of the uterine cavity. The tube passes laterally and
superiorly, and consists of four main parts: intramural, isthmus, ampulla
and fimbria (see Fig. 77.22). The intramural part is 0.7 mm wide and
1 cm long, and lies within the myometrium. It is continuous laterally
with the isthmus, which is 1–5 mm wide and 3 cm long; it is rounded,
muscular and firm. The isthmus is continuous laterally with the
ampulla, the widest portion of the tube with a maximum luminal
diameter of 1 cm. The ampulla is 5 cm long and has a thin wall and a
tortuously folded luminal surface. Typically, fertilization takes place in
its lumen. The ampulla opens into the trumpet-shaped infundibulum
at the abdominal os. Fimbriae, numerous mucosal finger-like folds
1 mm wide, are attached to the ends of the infundibulum and extend
from its inner circumference beyond the muscular wall of the tube. One
of these, the ovarian fimbria, is longer and more deeply grooved than
the others, and is typically applied to the tubal pole of the ovary. At the
time of ovulation, the fimbriae swell and extend as a result of engorgement of the vessels in the lamina propria, which aids capture of the
released oocyte. All fimbriae are covered, like the mucosal lining
throughout the tube, by a ciliated epithelium whose cilia beat towards the ampulla.
Vascular supply and lymphatic drainage
Arteries
The blood supply to the uterine tubes is derived from ovarian and uterine arteries (see Fig. 77.21A). The lateral third of the tube is supplied by the ovarian artery, which continues in the mesosalpinx to anastomose with branches from the uterine artery. The medial two-thirds of the tube are supplied by the uterine artery.














Nhận xét
Đăng nhận xét