Female reproductive system
BS. Nguyễn Hồng Anh
The onset of menstruation (menarche) occurs between the tenth and seventeenth years of life, with a peak between 12 and 14 years. The uterus, ovaries and vagina undergo changes during the menstrual cycle, which normally lasts approximately 28 days. A follicle begins a period of development in the ovary during the first days of the cycle, and matures and ruptures mid-cycle (approximately day 14 of a 28-day cycle) to release a secondary oocyte. The wall of the follicle is then transformed into the corpus luteum, which secretes progesterone (luteal phase). About 10 days after ovulation, the corpus luteum begins to regress, then ceases to function and is replaced by fibrous tissue. The breakdown of the endometrium that follows this cessation of function is due to the reducing levels of progesterone and oestrogen as the corpus luteum degenerates.
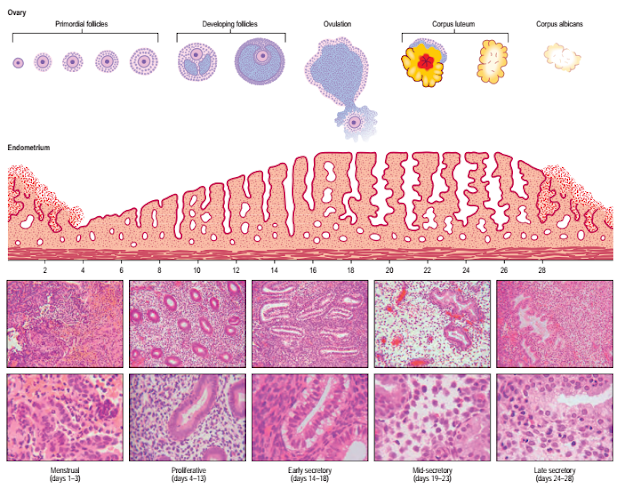
The changes that occur in the endometrium during the menstrual cycle may be divided into three phases: proliferative, secretory and menstrual (Fig. 77.37).
PROLIFERATIVE PHASE
In the early proliferative phase, even before the menstrual flow ceases, the epithelium from the persisting basal parts of the uterine glands grows over the surface of the endometrium, which has been denuded by menstruation. Re-epithelialization is complete 5–6 days after the start of menstruation. Initially, the tissue is only 1–2 mm thick and is lined by low cuboidal epithelium. The glands are straight and narrow, and their lining cells are short columnar. The apical cell surface contains microvilli; some cells are ciliated. The stroma is dense and contains small numbers of lymphocytes. During days 10–12 of the proliferative phase, the endometrium thickens. Cells divide in response to rising levels of oestrogen, which is produced by the ovary and which acts through receptors present on both the stromal and the epithelial cells.
The glands become tortuous and their lining epithelial cells become tall columnar in nature (Speroff and Fritz 2004).
SECRETORY PHASE
The secretory phase coincides with the luteal phase of the ovarian cycle. The endometrial changes are driven by progesterone and oestrogen, secreted by the corpus luteum. Steroid receptors in the endometrium activate a programme of new gene expression that produces, in the following 7 days, a highly regulated sequence of differentiative events, presumably required to prepare the tissue for blastocyst implantation.
Part of the response is direct, but there is evidence that some of the effects may be mediated through growth factors (Speroff and Fritz 2004). The first morphological effects of progesterone are evident 24–36 hours after ovulation (which occurs approximately 14 days before the next menstrual flow). In the early secretory phase, glycogen masses accumulate in the basal cytoplasm of the epithelial cells lining the glands, where they are often associated with lipid. Giant mitochondria appear and are associated with semi-rough endoplasmic reticulum.
There is an obvious increased polarization of the gland cells: nuclei are displaced towards the centre of the cells, and Golgi apparatus and secretory vesicles accumulate in the supranuclear cytoplasm. The nuclear channel system is prominent and nuclei enlarge. Nascent secretory products may be detected immunohistochemically within the cells.
Progestational effects on the stroma (known as the decidual reaction) are also evident in the early secretory phase. Nuclear enlargement occurs and the packing density of the resident stromal cells increases, due, in part, to the increase in volume of gland lumina and onset of secretory activity in the epithelial compartment.
By the mid-secretory phase, the endometrium may be up to 6 mm deep. The basal epithelial glycogen mass is progressively transferred to the apical cytoplasm, and nuclei return to the cell bases. The Golgi apparatus becomes dilated and products, including glycogen, mucin and other glycoproteins, are released from the glandular epithelium into the lumen by a combination of apocrine and exocrine mechanisms; this activity reaches a maximum 6 days after ovulation. These secretory changes are considerably less pronounced in the basal gland cells and the luminal epithelium than in the glandular cell population of the stratum functionalis. There is a notable stromal oedema and a corresponding decrease in the density of collagen fibrils. At the same time, the endoplasmic reticulum and Golgi apparatus become more prominent; there is evidence for collagen synthesis and degradation, presumably reflecting ongoing matrix remodelling. In the late secretory phase, glandular secretory activity declines.
Decidual differentiation occurs in the superficial stromal cells that surround blood vessels; this transformation includes nuclear rounding and an increased cytoplasmic volume, reflecting an increase in and dilation of the rough endoplasmic reticulum and Golgi systems, and cytoplasmic accumulation of lipid droplets and glycogen. The cells begin to produce basal lamina components, including laminin and type IV collagen.
Ultrasound is frequently used in the clinical evaluation of the endometrium. In the early secretory phase, the endometrium is identified as a thin echogenic line, a consequence of specular reflection from the interface between opposing surfaces of endometrium. During the late proliferative phase, the endometrium appears as a triple layer: a central echogenic line (due to the apposed endometrial surfaces), surrounded by a thicker hypoechoic functional layer, and bounded by an outer echogenic basal layer. During the secretory phase, the functional layer surrounding the echogenic line becomes more hyperechoic as a result of the increased mucus and glycogen within the glands and the increased number of interfaces caused by the development of tortuous spiral arteries (Fig. 77.38).
MENSTRUAL PHASE
Prior to menstruation, three layers can be recognized in the endometrium: the strata compactum, spongiosum and basale. In the stratum compactum, which is next to the free surface, the necks of the glands are only slightly expanded and the stromal cells show a distinct decidual reaction. In the stratum spongiosum, the uterine glands are tortuous, dilated and, ultimately, only separated from one another by a small amount of interglandular tissue. The stratum basalis, next to the uterine muscle, is thin and contains the tips of the uterine glands embedded in an unaltered stroma
Fig. 77.37 Stages in the menstrual cycle with concomitant changes in the endometrium. Note that developing antral follicles are selected from cohorts of follicles recruited in earlier cycles. The ten lower panels are histological sections of endometrium at the cycle times indicated (low magnification on top and high magnification below in each case).
Fig. 77.38 Transvaginal ultrasound of the uterus, showing the triplelayered endometrium. The central echogenic line is produced by the interface of the opposing surfaces of the endometrium. The functional layer of the endometrium surrounding the echogenic line is hypoechoic on the left, in keeping with late proliferative phase, but is becoming more hyperechoic on the right, indicating the early secretory phase.
The two upper strata are often grouped together as the functional layer, stratum functionalis, of the endometrium; the lower (basal) layer is called the stratum basalis. As regression of the corpus luteum occurs, those parts of the stroma showing a decidual reaction, together with the glandular epithelium, undergo degenerative changes and the endometrium often diminishes in thickness. Blood escapes from the superficial vessels of the endometrium, forming small haematomata beneath the surface epithelium (see below). The stratum functionalis, next to the free surface, is shed piecemeal, leaving mainly the stratum basalis, adjacent to the uterine muscle; 65–75% of the thickness of the endometrium may be shed. Blood and necrotic endometrium then begin to appear in the uterine lumen, to be discharged from the uterus through the vagina as the menstrual flow, which usually lasts 3–6 days.
The amount of tissue lost is variable, but usually the stratum compactum and most of the spongiosum are desquamated.
VASCULAR CHANGES WITHIN THE UTERUS DURING
THE MENSTRUAL CYCLE
The vascular bed of the endometrium undergoes significant changes during the menstrual cycle. The arteries to the endometrium arise from a myometrial plexus and consist of short, straight vessels to the basal portion of the endometrium, and more muscular spiral arteries to its superficial two-thirds. The venous drainage, consisting of narrow perpendicular vessels that anastomose by cross-branches, is common to both the superficial and the basal layers of the endometrium. The capillary bed consists of an endothelium with a basal lamina that is discontinuous in the proliferative phase, but becomes more distinct by the mid-secretory phase. Pericytes are present, some of which resemble smooth muscle cells, and these are sometimes enclosed within the basal lamina. The pericytes make contact with the endothelial cells by means of cytoplasmic extensions that project through the basal lamina.
Enlargement of the pericytes starts in the early secretory phase, and leads to a conspicuous cuff of cells in the mid- and late secretory phases. The arterial supply to the basal part of the endometrium remains unchanged during the menstrual cycle. However, the spiral arteries to the superficial strata lengthen disproportionately. They become increasingly coiled and their tips approach the uterine epithelium more closely.
This leads to a slowing of the circulation in the superficial strata with some vasodilation. Immediately before the menstrual flow, these vessels begin to constrict intermittently, causing stasis of the blood and anaemia of the superficial strata. During the periods of relaxation of the vessels, blood escapes from the devitalized capillaries and veins, causing the menstrual blood loss. The breakdown of the endometrium is a consequence of the reducing levels of progesterone and oestrogen as the corpus luteum degenerates.
PREGNANCY AND PARTURITION
During pregnancy, many morphological changes occur in the female reproductive system and associated abdominal structures. The uterus enlarges to accommodate the developing fetus and placenta, and various alterations take place in the pelvic walls, floor and contents that allow for this expansion, and which anticipate parturition. At the end of gestation, dramatic changes occur that facilitate the passage of the baby through the birth canal and, subsequently, allow the pelvic organs to return to the non-pregnant state (involution). On average, human gestation is approximately 280 days (or 40 weeks) from the date of the last menstrual period (LMP) (Ch. 14).
However, as the menstrual history can often be inaccurate due to cycle irregularity or inaccurate recollection, an accurate gestational age can be calculated by ultrasound measurement of the fetal crown–rump length in the first trimester (Fig. 77.39). A discrepancy of more than 5–7 days from the LMP-estimated age is usually cause to revise the estimated date of confinement. For women presenting after the first trimester, gestational dating can be based on other sonographically obtained fetal biometric measurements, such as the fetal biparietal diameter, head circumference and femur length, although precision gradually decreases later in pregnancy (see Fig. 14.4). The increased accuracy of gestational dating using sonographic fetal biometry rather than menstrual history alone has significantly reduced the number of pregnancies requiring induction of labour for post-term women.
UTERUS IN PREGNANCY
Fig. 77.39 Transabdominal ultrasound at 12 weeks, showing
measurement of the crown–rump length to calculate the gestational age.
The function of the uterus in pregnancy is to retain the developing fetus and to provide a protected environment until a stage at which the fetus is capable of surviving ex utero. The uterus must grow, facilitate delivery of the fetus and then involute.
The uterus grows dramatically during pregnancy, increasing in weight from about 50 g at the beginning of pregnancy to up to 1 kg at term. Most of the weight gain is the result of increased vascularity and fluid retention in the myometrium. The increased growth of the uterine wall is driven by a combination of mechanical stretching and endocrine input. The mechanical load that increasing gestation imposes on the uterine wall induces hypertrophy of uterine smooth muscle cells and is the major stimulus that increases smooth muscle mass. Some hyperplasia occurs early on in pregnancy, mainly from the growth of the media of the myometrial arteries and veins. The myometrium is relatively unresponsive to additional endocrine stimulation during most of pregnancy, a relative quiescence that is, in part, attributed to progesterone
However, a number of growth factors, e.g. insulin-like growth factor-1 (IGF-1), have been identified, which interact with oestrogen in promoting uterine growth. The myometrium thins with advancing gestation from 2–3 cm thick in early pregnancy to 1–2 cm at term.
The upper third of the cervix (isthmus) is gradually taken up into the uterine body during the second month to form the ‘lower segment’ (Fig. 77.40). The isthmus hypertrophies like the uterine body during the first trimester and triples in length to about 3 cm. From the second trimester, the wall of the isthmus and that of the body are the same thickness and their junction is no longer visible externally. This condition persists until the middle of the third trimester, when the lower uterine segment thins considerably to less than 1 cm in thickness. The thicker myometrium above this transition is the main contractile portion of the uterus that will generate the expulsive forces during labour; the thin lower uterine segment, which begins just below the vesico-uterine pouch and is thought to correspond to the level of the anatomical internal os, is more compliant and allows for the descent of the fetus in late pregnancy and during labour. As the lower uterine segment is thinner and less vascular than the upper uterus, it is the preferred site of incision during a caesarean section. In addition, because it is less contractile, there is a lower risk of uterine rupture in subsequent pregnancies, compared with an incision made in the body of the uterus during a ‘classic’ caesarean section.
Fig. 77.40 A frontal view of the uterus, showing the location and extent of the body, isthmus and cervix in the non-gravid and gravid uterus at different stages in gestation. The isthmus forms the lower uterine segment with advancing gestation.
Fig. 77.41 Transvaginal ultrasound at 36 weeks. A, Measurement of cervical length. B, Posterior placenta praevia 18.5 mm from the internal os. C, A placenta completely covering the internal cervical os, consistent with a complete placenta praevia.
The growing uterus generally emerges from the pelvis by the twelfth week of pregnancy and can be palpated on the maternal abdomen just above the pubic symphysis. By the twentieth week of pregnancy, the uterine fundus is at the level of the umbilicus and reaches the xiphisternum by 36 weeks. After 24 weeks of gestation, the distance between the pubic symphysis and the uterine fundus generally corresponds to the number of gestational weeks and is often used in clinical care as a screening method to detect a pregnancy that is measuring suspiciously larger or smaller than expected. If there is more than a 2 cm discrepancy, a more accurate, sonographic assessment of fetal size and amniotic fluid volume is indicated.
Cervix and pregnancy
During pregnancy, the uterine cervix is a relatively rigid, fibromuscular structure that retains the developing fetus within the uterus. The rigidity of the cervix appears to be related to the orientation of its collagen fibres within a regular connective tissue matrix. The cervix gradually softens and shortens in the weeks preceding labour. During active labour, the cervix dilates to allow the fetus to descend through the birth canal.
The exact processes that allow softening, effacement and dilation of the cervix are unclear, but are believed to include remodelling of the connective tissue matrix, probably mediated by an increase in the activity of enzymes such as matrix metalloproteinase 1, a reduction in collagen concentration, a significant increase in the water content in the cervix, an infiltration of macrophages and neutrophils, and an increased level of apoptosis.
Transvaginal ultrasound examination allows for a robust evaluation of the cervix during pregnancy. Cervical length, dilation of the internal os and bulging of the membranes into the canal can all be assessed
(Fig. 77.41). Detection of a shortened cervical length in mid-gestation using transvaginal ultrasound is a strong risk factor for premature delivery and has been used clinically to identify women at high risk of preterm delivery in order to plan appropriate management (Fonseca et al 2007, Hassan et al 2011, Owen et al 2009). Emerging sonographic and MRI technologies are being used to investigate both the biomechanical properties and the microstructure of the cervix in pregnancy and promise to shed light on the poorly understood mechanisms involved in both normal and pathological cervical shortening in human pregnancy (Feltovich et al 2012, House et al 2013).
Relations of the uterus in pregnancy
With uterine expansion, the ovaries and uterine tubes are displaced upwards and laterally. The round ligaments become hypertrophied and their course from the cornual regions of the uterus down to the internal inguinal ring becomes more vertical. The broad ligament tends to open out to accommodate the massive increase in the sizes of the uterine and ovarian vessels. The uterine veins, in particular, can reach about 1 cm in diameter and they appear to act as a significant reservoir for blood during uterine contractions. Lymphatics and nerves expand their territories (the significance of this increased innervation is not clear because paraplegic women are able to labour normally, albeit painlessly). Later in pregnancy, the increase in intra-abdominal pressure produced by the gravid uterus may produce eversion of the umbilicus. On the skin over the abdomen, a combination of stretching and hormonal changes may produce stretch marks (striae gravidarum). In multiparous patients, diastasis (diverification) of right and left rectus abdominis may occur to accommodate the enlarging uterine fundus further. In the supine position, the gravid uterus can cause aortocaval compression, leading to reduced venous return to the heart and decreased cardiac output. In some women, this can lead to symptomatic hypotension and symptoms of nausea and faintness. These symptoms are minimized if the woman lies on her left side, thereby deviating the gravid uterus and reducing compression of the inferior vena cava.
The jejunum, ileum and transverse colon tend to be displaced upwards by the enlarging uterus, whereas the caecum and appendix are displaced to the right, and the sigmoid colon posteriorly and to the left. Upward and lateral displacement of the appendix in later pregnancy can cause difficulties in the diagnosis of appendicitis. The ureters are pushed laterally by the enlarging uterus and, in late pregnancy, can be compressed at the level of the pelvic brim, resulting in hydronephrosis and loin pain. However, mild ureteric dilation is normal in pregnancy, and is caused by progesterone-induced relaxation of smooth muscle in the ureteric walls. The axis of the uterus is often dextrorotated by the presence of the sigmoid colon; this rotation must be considered when performing a hysterotomy at the time of caesarean section to avoid injuring the large uterine vessels located bilaterally.
PLACENTAL DEVELOPMENT
Fig. 77.42 A sagittal T2-weighted scan of a twin pregnancy with a placenta praevia overlying the internal cervical os.
Fig. 77.43 Doppler examination of the maternal and fetal circulation in pregnancy. A pulsed Doppler showing normal waveforms in the umbilical arteries at 28 weeks.
Placental attachment to the uterus occurs at the point where the blastocyst becomes embedded, and is determined by a multitude of molecular signalling pathways. In early pregnancy, the placental disc occupies a large proportion of the uterine cavity and will often appear to be situated near the internal os. In the majority of cases, growth and stretching of the uterus will usually draw the placenta upwards, away from the cervix, by the end of pregnancy.
In about 1% of pregnancies, the position of the placenta will remain over, or in close proximity to, the internal cervical os at the end of pregnancy (see Fig. 77.41B; Fig. 77.42). This condition is called placenta praevia and can be associated with vaginal bleeding during pregnancy and labour. If the placenta covers the internal os, or the lower edge is less than 1–2 cm from the internal os, delivery by caesarean section is generally indicated.
Normal trophoblastic development includes the remodelling of maternal spiral arterioles to allow for low-resistance flow into the intervillous space. Thus, the uteroplacental vasculature is a low-resistance, high-capacitance system. Antenatal ultrasound Doppler velocimetry can be used during pregnancy to evaluate the resistance to flow in both the uterine and the umbilical arteries, and may be helpful in the management of high-risk pregnancies (Fig. 77.43).
Abnormally invasive placentation can occur when trophoblastic tissue invades through the decidua basalis and reaches the myometrium; this can lead to a morbidly adherent placenta and significant maternal morbidity. This condition, placenta accreta, occurs in approximately 3 per 1000 births. When the trophoblastic tissue invades the myometrium, it is referred to as placenta increta; if the placental tissue reaches the uterine serosa, it is called a placenta percreta. In extreme cases, a placenta percreta can also invade into the maternal bladder mucosa. These conditions often require a hysterectomy. Prior uterine surgery, especially multiple prior caesarean sections, is the most significant risk factor
(Fig. 77.44
Fig. 77.44 A gross photograph taken at the time of caesarean section in a patient with a placenta accreta. Note the hypervascularity caused by the placental tissue invading into, and almost through, the myometrial layer of the lower uterine segment, which is still covered with its serosal layer.
LABOUR
The onset of labour is defined as the combination of regular uterine contractions that are of sufficient intensity to produce progressive effacement and dilation of the cervix. The process of labour is described in three main stages.
First stage
The first stage of labour is the period during which the cervix progressively dilates until the fetus is able to descend into the birth canal and there is no longer any cervix palpable on vaginal examination. There is little change in the uterine volume because myometrial contractions are isometric, and so there is minimal shortening of the muscle fibres.
Progress is determined by the equilibrium between forces generated by myometrial contractions, especially from the fundus, and the resistance of the cervix. second stage The second stage of labour begins once the cervix is fully dilated, and ends with the delivery of the fetus. Cervical resistance is lost and myometrial activity results in isometric contractions that aid descent of the fetus in the birth canal. The head of the fetus usually enters the pelvis with the occiput facing laterally. As the head descends further, the occiput contacts the gutter-shaped pelvic floor formed by levator ani and this promotes flexion and rotation of the occiput to the anterior position. With further descent, the occiput escapes under the pubic symphysis and the head is born by extension. At this point, the head of the fetus regains its normal relationship with its shoulders, and slight rotation (or restitution) of the head is seen. Further external rotation occurs as the leading shoulder is directed medially by the maternal pelvic floor. The body of the fetus is now born by lateral flexion as one shoulder slips underneath the symphysis and the posterior shoulder is drawn over the frenulum.
Third stage
The third stage of labour is the time from delivery of the fetus until delivery of the placenta. Prior to separation of the placenta, a large proportion of the mother’s cardiac output passes through the uterine circulation. After separation in the third stage of labour, maternal exsanguination is only prevented by marked uterine contraction; the crisscrossing myometrial fibres act as a tourniquet, restricting blood flow to the area that was the placental site. This process is usually expedited clinically by the administration of oxytocic drugs in an attempt to limit maternal blood loss. Any condition that predisposes to poor uterine contraction, such as retained placental tissue, will increase the likelihood of postpartum haemorrhage.
Placenta after delivery
Separation of the placenta from the uterine wall takes place along the plane of the stratum spongiosum and extends beyond the placental area, detaching the villous placenta, with associated fibrinoid matrix and small amounts of decidua basalis; the chorio-amnion, together with a superficial layer of the fused decidua capsularis; and the decidua parietalis. When the placenta and membranes have been expelled, a thin layer of stratum spongiosum is left lining the uterus; it soon degenerates and is cast off in the early part of the puerperium. A new epithelial lining is regenerated from the remaining stratum basalis. The expelled placenta is a flattened discoid mass with an approximately circular or oval outline (Fig. 77.45). It has an average volume of 500 ml (range 200–950 ml), a weight of 470 g (range 200–800 g), a diameter of 185 mm (range 150–200 mm), a thickness of 23 mm (range 10–40 mm), and a surface area of approximately 30,000 mm2. It is thickest at its centre (the original embryonic pole), and rapidly thins towards its periphery, where it continues as the chorion laeve. The size of the placenta correlates well with the birth weight of the neonate.
Fig. 77.45 The fetal surface of a recently delivered placenta. The spiral umbilical vessels in the umbilical cord and their radiating branches are seen through the transparent amnion. The maternal surface is exposed in the lower and right corner of the figure. Note the fringes of amnion and chorion, the majority of which have been cut away near the placental margin.
Macroscopically, the fetal or inner surface, covered by amnion, is smooth, shiny and transparent, so that the mottled appearance of the subjacent chorion, to which it is closely applied, can be seen. The umbilical cord is usually attached near the centre of the fetal surface, and branches of the umbilical vessels radiate out under the amnion from this point; the veins are deeper and larger than the arteries. The maternal surface of the placenta is finely granular and mapped into some 15–30 lobes by a series of fissures or grooves. The placental lobes, which are often somewhat loosely termed cotyledons, correspond, in large measure, to the major branches of the umbilical vessels. The grooves correspond to the bases of incomplete placental septa, which become increasingly prominent from the third month onwards. They extend from the maternal aspect of the intervillous space (the basal plate) towards the chorionic plate (see Fig. 9.5) but do not quite reach it. The septa are complex structures composed of components of the cytotrophoblastic shell and residual syncytium, together with maternally derived material, including decidual cells, occasional blood vessels and gland remnants, collagenous and fibrinoid extracellular matrix, and, in the later months of pregnancy, foci of degeneration.
In multiple pregnancies, the number of placentas is determined by the zygosity; for example, in twin gestations, dizygotic pregnancies will always have two placentas (dichorionic). Monozygotic pregnancies usually have a single placenta (monochorionic), but about one-third will have two placentas; the number is determined by the timing of splitting of the embryonic mass (see Ch. 8 and Fig. 8.9). If the split occurs within 3 days of fertilization, each fetus will have its own placenta and amniotic sac (dichorionic diamniotic); splitting after the third day following fertilization results in a monochorionic diamniotic pregnancy (two amniotic sacs) and vascular communication within the two placental circulations (Fig. 77.46); splitting after the ninth day results in a single placenta and single amniotic sac (monochorionic monoamniotic); and splitting after the twelfth day results in conjoined twins.
Fig. 77.46 A vascular cast from a monochorionic diamniotic twin pregnancy, showing vascular communications between both placentas. (Courtesy of Dr Ling Wee, University College London Hospital.)
Fig. 77.47 A battledore placenta showing the marginal insertion of the umbilical cord into the placenta. (Courtesy of Dr Michael Ashworth, Consultant Histopathologist, Great Ormond Street Hospital for Children, London.)
Fig. 77.48 Velamentous insertion of the umbilical cord, showing the cord inserting into the chorioamniotic membranes rather than the placental disc. (Courtesy of Dr Michael Ashworth, Consultant Histopathologist, Great Ormond Street Hospital for Children, London.)
(Fig. 77.48). They travel unprotected through the membranes to the placenta, and this puts the fetus at risk because compression or tearing of the vessels can disrupt blood flow to and from the fetus. This can be particularly problematic when the vessels present themselves across the cervical os, a condition called vaso praevia. An accessory (succenturiate) placental lobe is occasionally present, connected to the main organ by membranes and blood vessels. If it is inadvertently retained in utero after delivery of the main placental mass, it can cause postpartum haemorrhage or infection. Other variations include placenta membranacea, in which villous stems and their branches persist over the whole chorion; and placenta circumvallata, where the placental margin is undercut by a deep groove caused by an abnormally central insertion of the membranes (Fig. 77.49).
Fig. 77.49 Placenta circumvallata. A, The fetal surface and a thick ring of membranes on the fetal surface of the placenta. B, A section through the placenta, showing that the membranes insert central to the edge of the placental disc. (Courtesy of Dr Michael Ashworth, Consultant Histopathologist, Great Ormond Street Hospital for Children, London.)
Placental variations
The umbilical cord is usually attached near the centre of the placenta, although it can occasionally insert more laterally and closer to the placental margin; this condition is known as a battledore placenta (Fig. 77.47). Occasionally, the cord fails to reach the placenta itself and ends in the membranes as a velamentous insertion. When insertion of the cord is velamentous, the larger branches of the umbilical vessels traverse the membranes before they reach and ramify on the placenta













Nhận xét
Đăng nhận xét