Maternal Anatomy
BS. Nguyễn Hồng Anh
ANTERIOR ABDOMINAL WALL
■ Skin, Subcutaneous Layer, and Fascia
The anterior abdominal wall confines abdominal viscera, stretches to accommodate the expanding uterus, and provides surgical access to the internal reproductive organs. Thus, a comprehensive knowledge of its layered structure is required to surgically enter the peritoneal cavity. Langer lines describe the orientation of dermal fibers within the skin. In the anterior abdominal wall, they lie transversely.
As a result, vertical skin incisions sustain greater lateral tension and in general develop wider scars. In contrast, low transverse incisions, such as the Pfannenstiel, follow Langer lines and lead to superior cosmetic results.
The subcutaneous layer can be separated into a superficial, predominantly fatty layer—Camper fascia —and a deeper membranous layer—Scarpa fascia. Camper fascia continues onto the perineum to provide fatty substance to the mons pubis and labia majora and then to blend with the fat of the ischioanal fossa
Scarpa fascia continues inferiorly onto the perineum as Colles fascia, (p. 18). Beneath the subcutaneous layer, the anterior abdominal wall muscles are the midline rectus abdominis and pyramidalis muscles as well as the external oblique, internal oblique, and transversus abdominis muscles, which extend across the entire wall (Fig. 2-1). The fibrous aponeuroses of these three latter muscles form the primary fascia of the anterior abdominal wall. These fuse in the midline at the linea alba, which normally sonographically measures ≤15 mm wide below the umbilicus in nongravid women (Beer, 2009; Mota, 2018). An abnormally wide separation may reflect diastasis recti or ventral hernia.
These three aponeuroses also invest the rectus abdominis muscle as the rectus sheath. The construction of this sheath varies above and below a boundary, termed the arcuate line (see Fig. 2-1). Cephalad to this border, the aponeuroses invest the rectus abdominis bellies on both dorsal and ventral surfaces. Caudal to this line, all aponeuroses lie ventral or superficial of the rectus abdominis muscle, and only the thin transversalis fascia and peritoneum lie deep to the rectus (Loukas, 2008). This transition of rectus sheath composition can be seen best in the upper third of a midline vertical abdominal incision.
The paired small triangular pyramidalis muscles originate from the pubic crest and insert into the linea alba. These muscles lie atop the rectus abdominis muscle but beneath the anterior rectus sheath.
The umbilicus is covered by peritoneum, transversalis fascia, and skin and contains the umbilical ring. The ring is a defect in the linea alba through which the fetal umbilical vessels previously passed. The round ligament of the liver and the median umbilical and medial umbilical ligaments variably attach to the ring.
■ Transversalis Fascia and Peritoneum
Transversalis fascia is the thin fibrous tissue layer between the inner surface of the transversus abdominis muscle and the
FIGURE 2-1 Anterior abdominal wall anatomy. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
preperitoneal fat. Inferiorly, the transversalis fascia blends with the periosteum of the pubic bones.
The peritoneum contains a single layer of epithelial cells and a supporting connective tissue. The visceral peritoneum densely wraps around the abdominopelvic viscera, whereas the parietal peritoneum lines the inner surface of the abdominal walls. In the anterior abdominal wall, five elevations of parietal peritoneum converge toward the umbilicus and are known as umbilical ligaments.
The single median umbilical ligament is formed by the urachus, a fibrous fetal remnant that extends from the bladder apex to the umbilicus. Clinically, this ligament may be seen at laparotomy during peritoneal incision as a white, midline, fibrous cord.
The paired medial umbilical ligaments are formed by the obliterated fetal umbilical arteries. The paired lateral umbilical ligaments contain the patent inferior epigastric vessels (see Fig. 2-1).
■ Blood Supply and Lymphatics
The superficial epigastric, superficial circumflex iliac, and superficial external pudendal arteries arise from the femoral artery just below the inguinal ligament within the femoral triangle (see Fig. 2-1).
These vessels supply the skin and subcutaneous layers of the anterior abdominal wall and mons pubis. The superficial epigastric vessels are surgically important and course diagonally from their origin toward the umbilicus. With a low transverse skin incision, these vessels can usually be identified at a depth halfway between the skin and the anterior rectus sheath. They lie above the Scarpa fascia and several centimeters from the midline. Ideally, these vessels are identified and surgically occluded. Lymphatics run cephalad–caudad on the lower abdomen (fourani, 2015). Most channels lie in the dermis, and a smaller density is found between the Camper and Scarpa fascias (Friedman, 2015).
More deeply, the bilateral inferior epigastric artery and vein are respective branches of the external iliac vessels and supply anterior abdominal wall muscles, nerves, and fascia. Of surgical relevance, the inferior epigastric vessels initially course lateral to and then posterior to the rectus abdominis muscles, which they supply. A deep system of lymphatics follows these arteries (fourani, 2015).
Clinically, when a Maylard incision is used for cesarean delivery, the inferior epigastric vessels may be lacerated during muscle transection. Preventively, identification and surgical occlusion are preferable. These vessels rarely may rupture spontaneously or traumatically during pregnancy to create a rectus sheath hematoma (Eckhof, 2016; Gibbs, 2016).
On each side of the lower anterior abdominal wall, the Hesselbach triangle is the region bounded laterally by the inferior epigastric vessels, inferiorly by the inguinal ligament, and medially by the lateral border of the rectus abdominis muscle. Hernias that protrude through the abdominal wall in the boundaries of the Hesselbach triangle are termed direct inguinal hernias. In contrast, indirect inguinal hernias bulge through the deep inguinal ring, which lies lateral to this triangle, and enter the inguinal canal. Here, contents may exit out the superficial inguinal ring.
■ Innervation
The entire anterior abdominal wall is innervated by intercostal nerves (T7–11), the subcostal nerve (T12), and the iliohypogastric and the ilioinguinal nerves (L1). The intercostal and subcostal nerves are anterior rami of the thoracic spinal nerves and run along the lateral and then anterior abdominal wall between the transversus abdominis and internal oblique muscles (Fig. 2-2). This space, termed the transversus abdominis plane, can be used for postcesarean analgesia blockade (Chap. 25, p. 480) (Ng, 2018).
Near the rectus abdominis lateral borders, anterior branches of the intercostal and subcostal nerves travel superficially to pierce the posterior sheath, rectus muscle, and then anterior sheath to reach the skin. Thus, these nerve branches may be severed during a Pfannenstiel incision creation during the step in which the overlying anterior rectus sheath is separated from the rectus abdominis muscle (Chap. 30, p. 552).
FIGURE 2-2 Intercostal and subcostal nerves are the anterior rami of spinal nerves. In this figure, an intercostal nerve extends ventrally between the transversus abdominis and internal oblique muscles. During this path, the nerve gives rise to lateral and anterior cutaneous branches, which innervate the anterior abdominal wall. As shown by the inserted needle, the transversus abdominis plane (TAP) block takes advantage of this anatomy. (Modified with permission from Hawkins JL: Anesthesia for the pregnant woman.
In Yeomans ER, Hoffman BL, Gilstrap LC III, et al: Cunningham and Gilstraps’s Operative Obstetrics, 3rd ed. New York, NY: McGraw Hill; 2017.)
The iliohypogastric and ilioinguinal nerves originate from the anterior ramus of the first lumbar spinal nerve. They emerge lateral to the psoas major muscle and travel across the quadratus lumborum in the retroperitoneum and toward the iliac crest. Near this crest, both nerves pierce the transversus abdominis muscle and course ventromedially. At a site 2 to 3 cm medial to the anterior superior iliac spine, the nerves then pierce the internal oblique muscle and course superficial to it toward the midline (Whiteside, 2003). The iliohypogastric nerve perforates the external oblique aponeurosis near the lateral rectus border to provide sensation to the suprapubic skin (see Fig. 2-1). The ilioinguinal nerve in its course medially travels through the inguinal canal and exits through the superficial inguinal ring, which forms by splitting of external abdominal oblique aponeurosis fibers. This nerve supplies the skin of the mons pubis, upper labia majora, and medial upper thighs.
The ilioinguinal and iliohypogastric nerves can be severed during a low transverse incision or entrapped during closure, especially if incisions extend beyond the lateral borders of the rectus abdominis muscle (Rahn, 2010). These nerves carry sensory information only, and injury leads to loss of sensation within the areas supplied. Rarely, chronic pain may develop (Verhagen, 2018).
The T10 dermatome approximates the level of the umbilicus. Analgesia to this level is suitable for labor and vaginal birth. Regional analgesia for cesarean delivery or for puerperal sterilization ideally extends to S4.
PERINEUM
This diamond-shaped area has boundaries that mirror those of the bony pelvic outlet (p. 28). These are the pubic symphysis anteriorly, ischiopubic rami and ischial tuberosities anterolaterally, sacrotuberous ligaments posterolaterally, and coccyx posteriorly. An arbitrary line joining the ischial tuberosities divides the perineum into an anterior triangle, also called the urogenital triangle, and a posterior triangle, termed the anal triangle
(Fig. 2-3).
■ Vulva (Âm hộ)
Mons Pubis, Labia, and Clitoris
The vulva includes all structures visible externally in the urogenital triangle (tam giác niệu dục) . These are the mons pubis (gò mua), labia majora (môi lớn), labia minora (môi nhỏ), clitoris (âm vật), hymen (màng trinh), vestibule (tiền đình), urethral opening (lỗ niệu đạo), greater vestibular (Bartholin) glands (tuyến tiền đình lớn), minor vestibular glands (tuyến tiền đình nhỏ), and paraurethral glands (tuyến cận niệu đạo) (see Fig. 2-3). The vulva receives innervations and vascular support from the pudendal nerve (thần kinh thẹn) (p. 22).
The mons pubis is a fat-filled cushion overlying the symphysis pubis (xương mu). After puberty, the mons pubis skin is covered by hair that forms the triangular escutcheon (hình, huy hiệu), whose base (đáy) aligns (thẳng hàng) with the upper margin (mép) of the symphysis pubis. In men and some hirsute (rậm lông) women, the escutcheon extends farther onto the anterior (trước) abdominal wall toward the umbilicus (rốn) and thus is diamond shaped.
Labia majora (môi lớn) usually are 7 to 8 cm long, 2 to 3 cm wide, and 1 to 1.5 cm thick. They are continuous directly with the mons pubis superiorly (phía trên), and the round ligaments (dây chằng tròn) terminate (tận hết, chấm dứt) at their upper borders. Hair covers the labia majora, and apocrine, eccrine, and sebaceous glands (tuyến bã nhờn) are abundant (dồi dào). Beneath the skin, a dense (dày đặc) connective tissue (mô liên kết) layer is nearly void (không có) of muscular elements but is rich in elastic fibers and fat. This fat mass provides bulk to the labia majora and is supplied with a rich venous plexus (đám rối). During pregnancy, this plexus may develop varicosities (giãn) from pressure created by the growing uterus.
Each labium minus is a thin tissue fold that lies medial to each labium majus. The labia minora begin at the fourchette and extend superiorly, where each divides into two lamellae. From each side, the lower lamellae fuse to form the frenulum of the clitoris, and the upper lamellae merge to form the prepuce (see Fig. 2-3). The labia minora dimensions vary greatly among reproductive-aged women (Cao, 2015; Lykkebo, 2017). In one large study, the mean length was 4 cm (range 0.5 to 10 cm) and mean lateral span was 1.5 (range 0.1 to 6 cm) (Kreklau, 2018).
Structurally, the labia minora are composed of connective tissue with many small vessels, elastin fibers, but very little smooth muscle. Nerve fibers are numerous (Ginger, 2011a; Schober, 2015). The epithelia of the labia minora differ by location. Thinly keratinized stratified squamous epithelium covers the outer surface of each labium. On their inner surface, the lateral portion is covered by this same epithelium up to a demarcating line, termed the Hart line. Medial to this line, each labium is covered by squamous epithelium that is nonkeratinized. Labia minora lack hair follicles, eccrine glands, and apocrine glands but have many sebaceous glands (Wilkinson, 2011).
The clitoris is the principal female erogenous organ (Fig. 2-4). It is located beneath the prepuce, above the frenulum and urethra, and projects downward toward the vaginal opening. The clitoris rarely exceeds 2 cm in length and is composed of a glans, a corpus or body, and two crura. The glans is usually less than 0.5 cm in diameter and is covered by stratified squamous epithelium. Nerve bundles are prominent and correspond to the paired dorsal nerves of the clitoris (Jackson, 2019). The clitoral body contains two corpora cavernosa. Extending from the clitoral body, each corpus cavernosum diverges laterally to form a long, narrow crus. Each crus lies along the inferior surface of its respective ischiopubic ramus and deep to the ischiocavernosus muscle. The clitoral blood supply stems from branches of the internal pudendal artery. Specifically, the deep artery of the clitoris supplies the clitoral body, whereas the dorsal artery of the clitoris supplies the glans and prepuce.
Vestibule (tiền đình)
In adult women, the vestibule is an almond-shaped area that is enclosed by the Hart line laterally, the hymen medially, the clitoral frenulum anteriorly, and the fourchette posteriorly (see Fig. 2-3). The vestibule is usually perforated by six openings: the urethra, the vagina, two greater vestibular (Bartholin) gland ducts, and ducts of the two largest paraurethral glands—the Skene glands. The posterior portion of the vestibule between the fourchette and the vaginal opening is called the vestibular fossa (Hill, 2021). It is usually observed only in nulliparas.
FIGURE 2-3 Vulvar structures and subcutaneous layer of urogenital triangle. Inset: Vestibule boundaries and openings onto the vestibule. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
FIGURE 2-4 Clitoris and associated vulvar structures in superficial space of urogenital triangle. Inset: cross section through proximal body of clitoris. PM = perineal membrane; PS = pubic symphysis. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
The bilateral Bartholin glands are 0.5 to 1 cm in diameter. On their respective side, each lies inferior to the vestibular bulb (bulb of vestibule) and deep to the inferior end of the bulbospongiosus muscle (former bulbocavernosus muscle). A duct extends medially from each gland, measures 1.5 to 2 cm long, and opens distal to the hymeneal ring. One duct opens at 5 and the other at 7 o’clock on the vestibule. Following trauma or infection, either duct may swell and obstruct to form a cyst or, if infected, an abscess. In contrast, the minor vestibular glands are shallow glands lined by simple mucin-secreting epithelium and open along the Hart line.
The paraurethral glands are a collective arborization of glands whose numerous small ducts open into the urethra and predominantly along its entire inferior aspect (Costa, 2016; Huffman, 1948). The two largest are called Skene glands, and their ducts typically lie distally and near the urethral meatus.
Clinically, inflammation and duct obstruction of any of the paraurethral glands can lead to urethral diverticulum formation (Chap 56, p. 1007). The urethral opening or meatus is in the midline of the vestibule, 1 to 1.5 cm below the pubic arch, and a short distance above the vaginal opening.
■ Vagina and hymen
In adult women, the hymen is a thin membrane that surrounds all or much of the vaginal opening in an annular or crescent form. Mainly elastic fibers, collagen, and fine vessels compose the hymen. Nerve fibers are few and localize to its base. Both outer and inner hymeneal surfaces are covered by nonkeratinized stratified squamous epithelium (Mahran, 1964). The aperture of the intact hymen ranges in diameter from pinpoint to one that admits one or even two fingertips. As a rule, the hymen is torn at several sites during first coitus. However, identical tears may form by other penetration, for example, by tampons used during menstruation. The torn edges soon reepithelialize.
Proximal to the hymen, the vagina is a muscular tube that extends to the uterus and is interposed lengthwise between the bladder and the rectum (Fig. 2-5). Total vaginal length is 9 to 10 cm (Collins, 2017; Patnam, 2019). Anteriorly, the vagina is separated from the bladder and urethra by connective tissue—the vesicovaginal septum. Posteriorly, between the lower portion of the vagina and the rectum, similar tissues together form the rectovaginal septum. The upper fourth of the vagina is separated from the rectum by the rectouterine pouch, also called the cul-de-sac or pouch of Douglas (Balgobin, 2020).
Normally, the anterior and posterior walls of the vaginal lumen lie in contact. The vaginal apex is subdivided by the cervix into anterior, posterior, and two lateral fornices. Clinically, the internal pelvic organs usually can be palpated through the thin walls of these fornices.
The vaginal lining is composed of nonkeratinized stratified squamous epithelium and underlying lamina propria. In premenopausal women, this lining is thrown into numerous thin transverse ridges, known as rugae. Deep to this, a muscular layer contains smooth muscle, collagen, and elastin. Beneath this, an adventitial layer consists of collagen and elastin (Maldonado, 2020; Mazloomdoost, 2017). No true fascia separates the vagina from the bladder or from the rectum.
The vagina lacks glands. Instead, it is lubricated by a transudate that originates from the vaginal subepithelial capillary
FIGURE 2-5 Vagina and surrounding anatomy. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.) plexus and crosses the permeable epithelium (Kim, 2011). Due to increased vascularity during pregnancy, vaginal secretions are notably increased. At times, this may be confused with amnionic fluid leakage, and clinical diferentiation of these two is described in Chapter 22 (p. 426).
After birth-related epithelial trauma and healing, fragments of stratified epithelium occasionally are embedded beneath the vaginal surface. Similar to its native tissue, this buried epithelium continues to shed degenerated cells. As a result, epidermal inclusion cysts, which are filled with debris, may commonly form.
The vagina has an abundant vascular supply. The proximal portion is supplied by the cervical branch of the uterine artery and by the vaginal artery. The latter may variably arise from the uterine or inferior vesical artery or directly from the internal iliac artery. The middle rectal artery contributes supply to the posterior vaginal wall, whereas the distal walls receive contributions from the internal pudendal artery. At each level, vessels supplying each side of the vagina course medially across the anterior or posterior vaginal wall and form midline anastomoses.
An extensive venous plexus also surrounds the vagina and follows the course of the arteries. Lymphatics from the lower third, along with those of the vulva, drain primarily into the inguinal lymph nodes. Those from the remainder drain into the pelvic lymph nodes.
■ Perineal Body
This fibromuscular pyramidal mass lies in the midline at the junction between the urogenital and anal triangles (Figs. 2-3 and 2-5) (Oh, 1973; Soga, 2007). Clinically, it measures 3.5 to 5 cm in nulliparas from the posterior midline hymen to the mid-anal opening, which are standard pelvic organ prolapse-quantification (POP-Q) landmarks (Komorowski, 2014; Reimers, 2016). It lengthens slightly during pregnancy, and during second-stage labor, one study showed that it stretches >65 percent (Meriwether, 2016). It serves as the junction for several structures and provides significant perineal support (Shark, 2007). Superficially, the bulbospongiosus, superficial transverse perineal, and external anal sphincter muscles converge on the perineal body. More deeply, the perineal membrane, portions of the pubococcygeus muscle, and internal anal sphincter contribute (Larson, 2010).
The perineal body is incised during episiotomy incision and is torn with second-, third-, and fourth-degree lacerations. Data conflict as to whether a shorter perineal body length predisposes to higher-order lacerations (Deering, 2004; Dua, 2009; Hokenstad, 2015; Meriwether, 2016).
■ Urogenital Triangle (tam giác niệu dục)
Superficial Space
The urogenital triangle is bounded by the pubic rami superiorly, the ischial tuberosities laterally, and the superficial
FIGURE 2-6 Superficial space of the urogenital triangle and anal triangle. Structures on the left side of the image can be seen after removal of Colles fascia. Those on the right side are noted after removal of the superficial muscles of the urogenital triangle. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
transverse perineal muscles posteriorly (Fig. 2-6). It is divided into superficial and deep spaces by the perineal membrane. This membranous partition is a dense fibrous sheet that was previously known as the inferior fascia of the urogenital diaphragm. The perineal membrane attaches laterally to the ischiopubic rami, medially to the distal third of the urethra and vagina, posteriorly to the perineal body, and anteriorly to the arcuate ligament of the pubis (Stein, 2008). It lies at the depth of the hymeneal ring.
The superficial space has the perineal membrane as its deep wall and the Colles fascia as its superficial one. As noted earlier, the Colles fascia is the continuation of the Scarpa fascia onto the perineum. On the perineum, the Colles fascia securely attaches laterally to the pubic rami and fascia lata of the thigh, inferiorly to the superficial transverse perineal muscle and inferior border of the perineal membrane, and medially to the urethra, clitoris, and vagina. As such, the superficial space of the urogenital triangle is a relatively closed compartment.
This superficial space contains several important structures, which include the Bartholin glands, vestibular bulbs, clitoral body and crura, branches of the pudendal vessels and nerve, and the ischiocavernosus, bulbospongiosus, and superficial transverse perineal muscles. Of this muscular trio, the ischiocavernosus muscles each attach on their respective side to the medial aspect of the ischial tuberosity inferiorly and the ischiopubic ramus laterally. Anteriorly, each attaches to a clitoral crus and may help maintain clitoral erection by compressing the crus to obstruct venous drainage. Each bulbospongiosus muscle overlies a vestibular bulb and Bartholin gland. Anteriorly, they attach to the body of the clitoris and posteriorly, to the perineal body. Some more-recent anatomical studies instead describe that the bulbospongiosus muscles blend medially with the external anal sphincter (Baramee, 2020; Plochocki, 2016). The muscles constrict the vaginal lumen and aid release of Bartholin gland secretions. They also may contribute to clitoral erection by compressing the deep dorsal vein of the clitoris. The bulbospongiosus and ischiocavernosus muscles also pull the clitoris downward. Last, the superficial transverse perineal muscles are narrow strips that attach to the ischial tuberosities laterally and the perineal body medially.
Each vestibular bulb, also called bulb of vestibule, is an almond-shaped aggregation of veins that lie beneath the bulbospongiosus muscle on either side of the vestibule (Jeppson, 2018). They measure 5 cm long and 2 cm wide (Jackson, 2019).
The bulbs terminate inferiorly at approximately the middle of the vaginal opening and extend upward toward the clitoris. Their anterior extensions merge in the midline, below the clitoral body. During childbirth, veins in the vestibular bulb may be lacerated or may rupture to create a hematoma enclosed within the superficial space of the urogenital triangle.
Deep Space
This space lies above or deep to the perineal membrane and below the inferior investing fascia of the levator ani muscle. It contains portions of urethra and vagina, certain portions of internal pudendal artery branches, and muscles of the striated urogenital sphincter complex (Fig. 2-7).
Urethra (lỗ niệu đạo)
The female urethra measures 3 to 4 cm and originates within the bladder trigone (p. 27) (Hamner, 2018; Rahn, 2007). The distal two thirds of the urethra are fused with the anterior vaginal wall. The epithelial lining of the urethra is transitional epithelium at the urethrovesical junction. It then changes to a pseudostratified columnar along its proximal length but is a nonkeratinized stratified squamous epithelium distally (Carlile, 1987).
FIGURE 2-7 Deep space of urogenital triangle. Structures on the right side of the image can be seen after removal of the perineal membrane. Also shown are structures that attach to the perineal body: bulbospongiosus, superficial transverse perineal, external anal sphincter, and puboperinealis muscles as well as perineal membrane. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
The walls of the urethra consist of two layers of smooth muscle, an inner longitudinal and an outer circular. This is in turn surrounded by a circular layer of skeletal muscle referred to as the sphincter urethrae (see Fig. 2-7). Approximately at the junction of the middle and lower third of the urethra and within the deep perineal space, two straplike skeletal muscles called the sphincter urethrovaginalis and the compressor urethrae are found. Together with the sphincter urethrae, these constitute the external urethral sphincter (EUS), also called the striated urogenital sphincter complex (Mistry, 2020). The EUS supplies constant tonus and provides emergency reflex contraction to sustain continence.
Distal to the level of the perineal membrane, the walls of the urethra consist of fibrous tissue, serving to direct the urine stream. Here, the urethra has a prominent submucosal layer that is lined by hormonally sensitive stratified squamous epithelium. Within the submucosal layer on the dorsal (vaginal) surface of the urethra lie most paraurethral glands (p. 16).
The urethra receives its blood supply from branches of the inferior vesical, vaginal, or internal pudendal arteries. The pudendal nerve provides somatic innervation to the EUS. The urethra’s smooth muscle receives sympathetic and parasympathetic innervation from the inferior hypogastric plexus (p. 26) (Colleselli, 1998).
■ Pelvic Diaphragm
The pelvic diaphragm spans the pelvic outlet and lies deep to the urogenital and anal triangles (p. 17). This broad muscular floor provides substantial support to the pelvic viscera and is composed of the coccygeus and levator ani muscles (see Fig. 2-7). The levator ani muscle, in turn, contains the pubococcygeus, puborectalis, and iliococcygeus muscles. The pubococcygeus muscle is also termed the pubovisceral muscle and is subdivided based on points of insertion and function. These include the pubovaginalis, puboperinealis, and puboanalis muscles, which insert into the vagina, perineal body, and anus, respectively (Kearney, 2004).
Vaginal birth can damage the levator ani muscle or its innervation (DeLancey, 2003; Weidner, 2006). Evidence suggests that levator ani trauma may predispose women to later pelvic organ prolapse (Berger, 2018; Dietz, 2008). Current research efforts aim to minimize these injuries.
■ Anal Triangle
This triangle contains the anal canal, anal sphincter complex, and ischioanal fossae. The sphincter complex consists of the internal anal sphincter, external anal sphincter, and puborectalis muscle. The anal triangle also contains branches of the pudendal nerve and internal pudendal vessels.
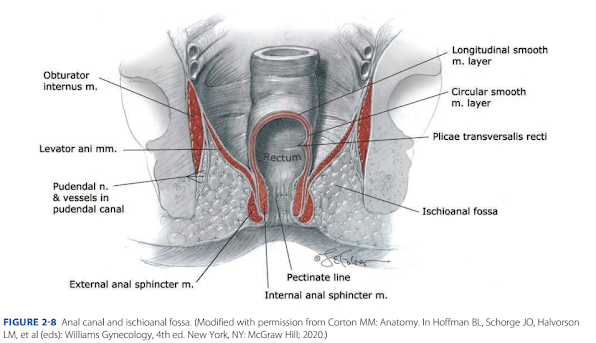
FIGURE 2-8 Anal canal and ischioanal fossa. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
Anal Canal
This is the distal continuation of the rectum. Canal mucosa is a columnar epithelium proximally. However, at the pectinate line, also termed dentate line, simple stratified squamous epithelium begins and continues to the anal verge (Fig. 2-8). The anal verge is defined as the transition point at which a keratin layer and skin adnexa join the squamous epithelium. Two definitions describe anal canal boundaries. The surgical anal canal begins at the levator ani muscle, ends at the anal verge, and measures 4 cm. The anatomical anal canal lies between the pectineal line and anal verge and is 2 cm long (Nivatvongs, 1981).
The anal canal has several tissue layers. Inner layers include the anal mucosa, the internal anal sphincter, and an intersphincteric space that contains continuation of the rectum’s longitudinal smooth muscle layer. An outer layer contains the external anal sphincter caudally and the puborectalis muscle cephalad.
Within the anal canal, three highly vascularized submucosal arteriovenous plexuses, termed anal cushions, aid complete closure of the canal and fecal continence when apposed. Increasing uterine size, excessive straining, and hard stool create raised pressure that ultimately leads to degeneration and subsequent laxity of the cushion’s supportive connective tissue base. These cushions then protrude into and downward through the anal canal. This leads to venous engorgement within the cushions— now termed hemorrhoids. Venous stasis results in inflammation, erosion of the cushion’s epithelium, and bleeding.
External hemorrhoids are those that arise distal to the pectinate line. They are covered by stratified squamous epithelium and receive sensory innervation from the inferior rectal nerve. Accordingly, pain and a palpable mass are typical complaints. Following resolution, a hemorrhoidal tag may remain and is composed of redundant anal skin and fibrotic tissue. In contrast, internal hemorrhoids are those that form above the pectinate line and are covered by insensitive anorectal mucosa. These may prolapse or bleed but rarely become painful unless they undergo thrombosis or necrosis.
Anal Sphincter Complex
Two sphincters surround the anal canal to provide fecal continence—the external and internal anal sphincters. Both lie near the vagina and may be torn during vaginal delivery. The internal anal sphincter (IAS) is a distal continuation of the rectal circular smooth muscle layer. It receives predominantly parasympathetic fibers, which pass through the pelvic splanchnic nerves. Along its length, this sphincter is supplied by the superior, middle, and inferior rectal arteries. The IAS contributes the bulk of anal canal resting pressure for fecal continence and relaxes prior to defecation. Te IAS measures 3 to 4 cm in length, and at its distal margin, it overlaps the external sphincter for 1 to 2 cm (DeLancey, 1997). The distal site at which this overlap ends, called the intersphincteric groove, is palpable on digital examination. It is 2 to 3 mm thick (Rociu, 2000).
In contrast, the external anal sphincter (EAS) is a striated muscle ring that anteriorly attaches to the perineal body and posteriorly connects to the coccyx via the anococcygeal ligament. The EAS measures 1.5 to 2.5 cm deep and 6 to 15 mm thick (Fenner, 1998; Stewart, 2018). The EAS is considered to have one or more subdivisions, although the precise composition is disputed (Lee, 2018). Often, a surrounding fibrous capsule is described, but this most likely is perineal body rather than a true EAS sheath (Maldonado, 2020).
FIGURE 2-9 Branches of pudendal nerve and artery. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
The EAS maintains a constant resting contraction to aid continence, provides additional squeeze pressure when continence is threatened, yet relaxes for defecation. The EAS receives blood supply from the inferior rectal artery, which is a branch of the internal pudendal artery. Somatic motor innervation derives from the inferior rectal branch of the pudendal nerve supply.
Clinically, the IAS and EAS may be involved in third- and fourth-degree lacerations during vaginal delivery, and reunion of these rings is integral to defect repair (Chap. 27, p. 511).
Ischioanal Fossae
These two fat-filled wedge-shaped spaces are found on either side of the anal canal and constitute the bulk of the anal triangle (Figs. 2-8 and 2-9). Formerly known also as ischiorectal fossae, each fossa has skin as its superficial base, whereas its deep border is formed by the levator ani muscle. Other borders include: laterally, the obturator internus muscle and ischial tuberosity; medially, the anal sphincter complex and levator ani muscle; posteriorly, the gluteus maximus muscle and sacrotuberous ligament; and anteriorly, the inferior border of the urogenital triangle.
The fat found within each fossa provides support to surrounding organs yet allows rectal distention during defecation and vaginal stretching during delivery. Clinically, injury to vessels in the anal triangle can lead to hematoma formation in the ischioanal fossa. Moreover, the two fossae communicate posteriorly through the deep postanal space. This connection lies between the pelvic floor muscles and anococcygeal ligament (Llauger, 1998). As a result, episiotomy infection or hematoma may extend from one fossa into the other.
■ Pudendal Nerve
This is formed from the anterior rami of S2–4 spinal nerves. It courses between the piriformis and coccygeus muscles and exits through the greater sciatic foramen at a location posterior to the sacrospinous ligament and just medial to the ischial spine (Maldonado, 2015). Thus, when injecting local anesthetic for a pudendal nerve block, the ischial spine serves as an identifiable landmark (Chap. 25, p. 471). The pudendal nerve then runs beneath the sacrospinous ligament and above the sacrotuberous ligament as it reenters the lesser sciatic foramen to course across the obturator internus muscle. Atop this muscle, the nerve lies within the pudendal canal, also known as the Alcock canal.
This space is formed by splitting of the obturator internus investing fascia (Shafk, 1999). In general, the pudendal nerve is relatively fixed as it courses behind the sacrospinous ligament and within the pudendal canal. Accordingly, it may be at risk of stretch injury during downward displacement of the pelvic floor during childbirth (Lien, 2005).
The pudendal nerve leaves this canal to enter the perineum and divides into three terminal branches (see Fig. 2-9). The first of these, the dorsal nerve of the clitoris, runs between the ischiocavernosus muscle and perineal membrane to supply the clitoral glans (Ginger, 2011b). Second, the perineal nerve runs superficial to the perineal membrane (Montoya, 2011). It divides into posterior labial branches and muscular branches, which serve the labial skin and the urogenital triangle muscles, respectively. Last, the inferior rectal branch runs through the ischioanal fossa to supply the external anal sphincter, the anal mucosa, and the perianal skin (Mahakkanukrauh, 2005).
The major blood supply to the perineum is from the internal pudendal artery, and its branches mirror the divisions of the pudendal nerve. Relevant to pudendal nerve blockade, the internal pudendal artery is the closest vascular structure, within 5 to 8 mm. It runs deep to the spine in most cases as it exits the greater sciatic foramen (Dueñas-Garcia, 2017; Roshanravan, 2007).
INTERNAL GENERATIVE ORGANS
■ Uterus
The nonpregnant uterus lies in the pelvic cavity between the bladder anteriorly and the rectum posteriorly. Anterior and posterior uterine walls are lined by serosa, that is, visceral peritoneum (Fig. 2-10). This peritoneum continues from the posterior wall to create the rectouterine space (see Fig. 2-5). It continues anteriorly to create the vesicouterine pouch. Clinically, during cesarean delivery, the peritoneum of the vesicouterine pouch is sharply incised, and the vesicocervical space is entered. This space is a well-defined loose connective tissue layer between the cervix and bladder (Balgobin, 2020). Dissection caudally within this space lifts the bladder safely of the cervix and lower uterine segment for hysterotomy and delivery (Chap. 30, p. 553).
The uterus is pear shaped and consists of two major but unequal parts. The upper, larger portion is the body or corpus, whereas the lower smaller cervix projects into the vagina. The isthmus is the union site oh these two. It is oh special obstetrical significance because it forms the lower uterine segment during pregnancy. At each superolateral margin of the body is a uterine cornu, from which a fallopian tube emerges. This area also contains the origins of the round and uteroovarian ligaments.
Between the points of fallopian tube insertion is the convex upper uterine segment termed the fundus. The bulk of the uterine body, but not the cervix, is muscle. The inner surfaces of the uterine walls lie almost in contact, and the intervening cavity forms a mere slit. The nulligravid uterus measures 6 to 8 cm in length compared with 9 to 10 cm in multiparas. The uterus averages 60 g and typically weighs more in parous women (Langlois, 1970; Sheikhazadi, 2010). Pregnancy stimulates remarkable uterine growth due to muscle fiber hypertrophy. The uterine fundus, a previously fattened
FIGURE 2-10 Uterus, adnexa, and associated anatomy. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.) convexity between tubal insertions, now becomes dome shaped.
Moreover, the round ligaments appear to insert at the junction of the middle and upper thirds of the organ. The fallopian tubes elongate, but the ovaries grossly appear unchanged.
Cervix
This portion of the uterus is cylindrical and has small apertures at each end—the internal os and external os. The endocervical canal runs through the cervix and connects these ora. The cervix is divided into upper and lower portions by the vagina’s attachment to its outer surface. The upper portion—the portio supravaginalis—begins at the internal os, which corresponds to the level of the vesicouterine peritoneum (see Figs. 2-5 and 2-10). The lower cervical portion protrudes into the vagina as the portio vaginalis.
Before childbirth, the external cervical os is a small, regular, oval opening. After labor, especially vaginal childbirth, the orifice is converted into a transverse slit that is divided such that there are the so-called anterior and posterior cervical lips.
If torn deeply during labor or delivery, the cervix may heal in such a manner that it appears irregular, nodular, or stellate (Fig. 36-1, p. 635). The cervical surface that radially surrounds the external os is called the ectocervix and is lined predominantly by nonkeratinized stratified squamous epithelium. In contrast, the endocervical canal is covered by a single layer of mucin-secreting columnar epithelium, which creates deep cleftlike infoldings or “glands.” Commonly during pregnancy, the endocervical epithelium moves out and onto the ectocervix in a physiological process termed eversion (Chap. 4, p. 53).
The cervical stroma is composed mainly of collagen, elastin, and proteoglycans, but very little smooth muscle. As described in Chapter 21 (p. 406), changes in the amount, composition, and orientation of these components lead to cervical ripening prior to labor. In early pregnancy, increased vascularity within the cervix stroma beneath the epithelium creates an ectocervical blue tint that is characteristic of the Chadwick sign. Cervical edema leads to softening—the Goodell sign, whereas isthmic softening is the Hegar sign.
Myometrium and Endometrium
Most of the uterus is composed of myometrium, which contains smooth muscle bundles united by connective tissue with many elastic fibers. Interlacing myometrial fibers surround myometrial vessels and contract to compress these. This anatomy allows hemostasis at the placental site during third-stage labor.
The uterine cavity is lined with endometrium, which is composed of an overlying epithelium, invaginating glands, and a supportive, vascular stroma. Throughout the menstrual cycle, the endometrium varies greatly (Chap. 5, p. 83). It is divided into a functionalis layer, which is sloughed with menses, and a basalis layer, which serves to regenerate the functionalis layer following each menses. During pregnancy, the endometrium undergoes dramatic hormonally driven alterations and is termed decidua.
Ligaments
Several ligaments extend from the uterine surface toward the pelvic sidewalls and include the round, broad, cardinal, and uterosacral ligaments (see Fig. 2-10). Despite their appellation, the round and broad ligaments provide no substantial uterine support, which contrasts with the cardinal and uterosacral ligaments.
The round ligament originates somewhat below and anterior to the origin of the fallopian tubes. Clinically, this orientation can aid tube identification during puerperal sterilization (Chap. 39, p. 682). Each round ligament extends laterally and down into the inguinal canal, through which it passes, to terminate in the upper portion of the ipsilateral labium majus. The artery to the round ligament, formerly the Sampson artery, is a small branch of the uterine artery and runs within the ligament. In nonpregnant women, the round ligament varies from 3 to 5 mm in diameter and is composed of smooth muscle bundles separated by fibrous tissue septa (Mahran, 1965). During pregnancy, these ligaments hypertrophy and increase appreciably in both length and diameter. Rare, round ligament varicosities can mimic an inguinal hernia, and color Doppler interrogation of the mass aids diagnosis (Yonggang, 2017).
The broad ligaments are two winglike structures that extend from the lateral uterine margins to the pelvic sidewalls. Each broad ligament consists of a double-layer drape of peritoneum. The anterior and posterior layers of this drape are termed the anterior and posterior leaves, respectively. In forming the broad ligament, this peritoneum folds over structures extending from each cornu. Peritoneum that folds over the fallopian tube is termed the mesosalpinx, that around the round ligament is the mesoteres, and that over the ovarian ligament is the mesovarium. Peritoneum that extends beneath the fimbriated end of the uterine tube toward the pelvic wall forms infundibulopelvic ligament, which is also the suspensory ligament of the ovary. This contains nerves and the ovarian vessels, and during pregnancy, these vessels, especially the venous plexuses, are dramatically enlarged. Specifically, the diameter of the ovarian vascular pedicle increases from 0.9 cm to reach 2.6 cm at term
(Hodgkinson, 1953).
The cardinal ligament—formerly the transverse cervical ligament or the Mackenrodt ligament—anchors medially to the uterus and upper vagina. The cardinal ligament is the thick base of the broad ligament. As such, during cesarean hysterectomy, sturdy clamps and suture are required for its transection and ligation.
Each uterosacral ligament originates with a posterolateral attachment to the supravaginal portion of the cervix and inserts into the fascia over the sacrum, with some variations (Ramanah, 2012; Umek, 2004). These ligaments are composed of connective tissue, small neurovascular bundles, and some smooth muscle. Covered by peritoneum, these ligaments form the lateral boundaries of the rectouterine space.
The term parametrium describes the connective tissues adjacent and lateral to the uterus within the broad ligament. Paracervical tissues are those adjacent to the cervix, whereas paracolpium is tissue lateral to the vaginal walls.
■ Pelvic Blood Supply
During pregnancy, uterine vasculature, which is supplied principally from the uterine and ovarian arteries, markedly hypertrophies (see Fig. 2-10). The uterine artery is a main branch of the internal iliac artery (prior hypogastric artery) and enters the base of the broad ligament. From its origin, the uterine artery courses medially to the lateral side of the uterus. Approximately 2 cm lateral to the cervix, the uterine artery crosses over the ureter. This proximity is important surgically, as the ureter may be injured or ligated during hysterectomy when the uterine vessels are clamped and ligated.
Once the uterine artery reaches the cervix, it divides. The smaller cervicovaginal artery supplies blood to the lower cervix and upper vagina. Instead, the main uterine artery branch turns abruptly upward and travels cephalad along the lateral margin of the uterus. Along this path, the uterine artery gives rise to the arcuate arteries. Indicated by the name, each branch arches across either the anterior or posterior uterine wall and courses within the myometrium just beneath the serosal surface. Arcuate vessels rom each side anastomose at the uterine midline.
From the arcuate arteries, radial arteries originate at right angles and travel inward through the myometrium. Upon entering the endometrium/decidua, they branch to become either basal arteries or spiral arteries. The coiled spiral arteries supply the functionalis layer. The basal arteries, also called the straight arteries, extend only into the basalis layer.
As the uterine artery courses cephalad, it gives rise to the artery of the round ligament. At the each cornu, the uterine artery divides into three terminal branches. First, the tubal branch makes its way through the mesosalpinx and supplies part of the fallopian tube; whereas the fundal branch penetrates the uppermost uterus. Third, the ovarian branch of the uterine artery forms an anastomosis with the terminal branch of the ovarian artery.
The ovarian artery is a direct branch of the aorta and enters the broad ligament through the infundibulopelvic ligament. At the ovarian hilum, it divides into smaller branches that enter the ovary. As the ovarian artery runs along the hilum, it also sends several branches through the mesosalpinx to supply the fallopian tubes. Its main stem, however, traverses the entire length of the broad ligament toward the uterine cornu. Here, it forms an anastomosis with the ovarian branch of the uterine artery. This dual uterine blood supply creates a vascular reserve to prevent uterine ischemia if ligation of the uterine or internal iliac artery is performed to control postpartum hemorrhage.
Uterine veins accompany their respective arteries. As such, the arcuate veins unite to form the uterine vein, which empties into the internal iliac vein and then the common iliac vein. Some of the blood from the upper uterus, the ovary, and the upper part of the broad ligament is collected by several veins. These terminate in the ovarian vein. From here, the right ovarian vein empties into the vena cava. The left ovarian vein empties into the left renal vein.
Blood supply to the pelvis is predominantly provided by branches of the internal iliac artery (Fig. 2-11). These branches are organized into anterior and posterior divisions, and subsequent branches are highly variable between individuals. The anterior division provides blood supply to the pelvic organs and perineum and includes the inferior gluteal, internal pudendal, middle rectal, vaginal, uterine, and obturator arteries, as well as the umbilical artery and its continuation as the superior vesical artery. The posterior division branches extend to the buttock and thigh and include the superior gluteal, lateral sacral, and iliolumbar arteries. For this reason, during internal iliac artery ligation, many advocate ligation distal to the posterior division to avoid compromise to the areas supplied by this division (Bleich, 2007).
FIGURE 2-11 Pelvic arteries. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
■ Pelvic Lymphatics
The lymphatic vessels from the uterine corpus generally empty into two nodal groups. Those from the cervix and lower uterine segment travel to the pelvic lymph nodes, which then drain into the paraaortic nodes. Vessels from the fundus, after joining lymphatics from the adnexa, directly terminate in the paraaortic lymph nodes. Vaginal lymphatic channels have extensive anastomoses. As a result, any node in the pelvis, groin, or anorectal area may drain any part of the vagina. Lymphatics of the vulva and distal vagina typically empty into the superficial inguinal nodal group. From here, lymph travels through the deep femoral lymphatics to the pelvic nodal groups.
■ Pelvic Innervation
As a brie review, the peripheral nervous system is divided into a somatic division, which innervates skeletal muscle, and an autonomic division, which innervates smooth muscle, cardiac muscle, and glands. Pelvic visceral innervation is predominantly autonomic, which is further divided into sympathetic and parasympathetic components.
Sympathetic innervation to pelvic viscera begins with the superior hypogastric plexus, also termed the presacral nerve (Fig. 2-12). This plexus is formed by sympathetic fibers arising from spinal levels N10 through L2. At the level of the sacral promontory, the superior hypogastric plexus divides into a right and a left hypogastric nerve, which run downward along their respective pelvic sidewalls (Ripperda, 2017).
FIGURE 2-12 Pelvic innervation. (Modified with permission from Corton MM: Anatomy. In Hoffman BL, Schorge JO, Halvorson LM, et al (eds): Williams Gynecology, 4th ed. New York, NY: McGraw Hill; 2020.)
In contrast, parasympathetic innervation to the pelvic organs derives from spinal levels S2 through S4. Anterior rami of the spinal nerves for those levels combine on each side to form the pelvic splanchnic nerves, also termed nervi erigentes. Blending of the two hypogastric nerves (sympathetic) and the two pelvic splanchnic nerves (parasympathetic) gives rise to the inferior hypogastric plexus, also termed the pelvic plexus. From here, fibers of this plexus extend to the bladder, uterus, upper vagina, and rectum (Ripperda, 2017; Spackman, 2007). Extensions of the inferior hypogastric plexus also reach the perineum to innervate the clitoris and vestibular bulbs (Montoya, 2011).
For the uterus, most of its afferent sensory fibers ascend through the inferior hypogastric plexus and enter the spinal cord via T10 through T12 and L1 spinal nerves. These transmit the painful stimuli of contractions to the central nervous system. For the cervix and upper part of the birth canal, sensory nerves pass through the pelvic splanchnic nerves to the second, third, and fourth sacral nerves. Last, those from the lower portion of the birth canal pass primarily through the pudendal nerve. Anesthetic blocks used during delivery target these levels of innervation.
■ Ovaries
Along the pelvic sidewall, each ovary usually rests in the ovarian fossa, which is a slight depression between the external and internal iliac vessels. During childbearing years, ovaries variably measure 2.5 to 5 cm in length, 1.5 to 3 cm in width, and 0.6 to 1.5 cm in thickness.
The ovarian ligament, also called the uteroovarian ligament, originates from the posterolateral uterine cornu and extends to the uterine pole of the ovary (see Fig. 2-10). Measuring a few centimeters long and 3 to 4 mm in diameter, this ligament is made up of muscle and connective tissue and is covered by peritoneum—the mesovarium. Blood supply reaches the ovary through the mesovarium to enter the ovarian hilum.
The ovary consists of an outer cortex and inner medulla. In young women, the cortex is smooth, white, and lined by a single layer of cuboidal epithelium. This epithelium is supported by an inner connective tissue condensation, the tunica albuginea. Beneath this, the ovarian cortex contains oocytes and developing follicles. The medulla is composed of loose connective tissue, numerous arteries and veins, and a small amount of smooth muscle.
The ovaries are supplied with both sympathetic and parasympathetic nerves. The sympathetic nerves are derived primarily from the ovarian plexus that accompanies the ovarian vessels and originates in the renal plexus. Others derive from the plexus that surrounds the ovarian branch of the uterine artery. Parasympathetic input is from the vagus nerve. Sensory aferents follow the ovarian artery and enter at T10 spinal cord level.
■ Fallopian Tubes
Each of these serpentine (ngoằn ngoèo) tubes extend laterally 8 to 14 cm from their respective (tương ứng) uterine cornu (sừng). Along their length, they contain an interstitial (kẽ) portion, isthmus (eo), ampulla (bóng), and infundibulum (phễu) (Fig. 2-13). Most proximal, the interstitial portion is embodied within the uterine muscular wall. Next, the narrow 2- to 3-mm wide isthmus widens gradually into the 5- to 8-mm wide ampulla. Last, the infundibulum is the funnel-shaped fimbriated distal extremity of the tube, which opens into the abdominal cavity. These latter three extrauterine portions are covered by the mesosalpinx, which is a superior extension of the broad ligament and described next.
In cross section, the uterine tube contains a mesosalpinx, myosalpinx, and endosalpinx. The outer of these, the mesosalpinx, is a single-cell mesothelial layer functioning as visceral peritoneum. In the myosalphinx, smooth muscle is arranged in an inner circular and an outer longitudinal layer. The tubal musculature undergoes rhythmic contractions constantly, the rate of which varies with cyclical ovarian hormonal changes.
FIGURE 2-13 The fallopian tube of an adult woman with cross-sectioned illustrations of the gross structure in several portions: (A) isthmus, (B) ampulla, and (C) infundibulum. Below these are photographs of corresponding histological sections. (Reproduced with permission from Dr. Kelley S. Carrick.) The endosalpinx (tubal mucosa) is a single layer of columnar epithelium made up of ciliated, secretory, and intercalary cells resting on a sparse lamina propria. Clinically, its close proximity to the underlying myosalphinx contributes to easy invasion by ectopic trophoblast. The tubal mucosa is arranged in longitudinal folds that become progressively more complex toward the fimbria.
In the ampulla, the lumen is occupied almost completely by the arborescent mucosa. The current produced by the tubal cilia flows toward the uterine cavity. Tubal peristalsis created by cilia and muscular layer contraction aids ovum transport (Croxatto, 2002).
The tubes are supplied richly with elastic tissue, blood vessels, and lymphatics. Their sympathetic innervation is extensive, in contrast to their parasympathetic innervation. This nerve supply derives partly from the ovarian plexus and partly from the inferior hypogastric plexus. Sensory afferent fibers ascend to T10 spinal cord levels.
LOWER URINARY TRACT
■ Bladder
Anteriorly, the bladder rests against the inner surface of the pubic bones and then, as it fills, also against the anterior abdominal wall. Posteriorly, it rests against the vagina and cervix. The bladder is divided into a dome and a base approximately at the level of the ureteral orifices. The dome is thin walled and distensible, whereas the base is thicker and undergoes less distention during filling. The vesical trigone lies in the bladder base and contains both ureteral orifices and the internal urinary meatus. The urethral lumen begins at this meatus and then courses through the bladder base for less than 1 cm. This region where the urethral lumen traverses the bladder base is the bladder neck.
The bladder wall consists of coarse bundles of smooth muscle known as the detrusor muscle, which extends into the proximal part of the urethra. A submucosal layer intervenes between this detrusor muscle and the mucosa. Te bladder mucosa consists of transitional epithelium and underlying lamina propria.
The blood supply to the bladder arises from the superior vesical arteries, which are branches of the patent portion of the umbilical artery and supply the dome (see Fig. 2-11). The inferior vesical arteries supply the base and variably arise from either the umbilical, uterine, or vaginal artery (de foreigny, 2017). The nerve supply to the bladder arises from the inferior hypogastric plexus (see Fig. 2-12).
■ Ureter
As the ureter enters the pelvis, it crosses over the bifurcation of the common iliac artery and passes just medial to the ovarian vessels (see Fig. 2-10). As the ureter descends into the pelvis, it lies medial to the internal iliac branches and anterolateral to the uterosacral ligaments. The ureter then traverses through the cardinal ligament approximately 1 to 2 cm lateral to the cervix. Near the level of the uterine isthmus, it courses below the uterine artery and travels anteromedially toward the bladder base.
In this path, it runs close to the upper third of the anterior vaginal wall (Jackson, 2019; Rahn, 2007). Finally, the ureter enters the bladder and travels obliquely for approximately 1.5 cm before opening at the ureteral orifices.
The pelvic ureter receives blood supply from the vessels it passes: the common iliac, internal iliac, uterine, and superior vesical vessels. The ureter’s course runs medial to these vessels, and thus its blood supply reaches the ureter from lateral sources. This is important during ureteral isolation. Vascular anastomoses on the connective tissue sheath enveloping the ureter form a longitudinal network of vessels.
PELVIC SKELETAL ANATOMY
■ Pelvic Bones and Joints
The pelvis is composed of four bones—the sacrum, coccyx, and two innominate bones. Each innominate bone is formed by the fusion of three bones—the ilium, ischium, and pubis (Fig. 2-14). Both innominate bones are joined to the sacrum at the sacroiliac joint. Anteriorly, they are joined at the symphysis pubis. This consists of fibrocartilage and the superior and inferior pubic ligaments. The latter ligament is frequently designated the arcuate ligament of the pubis.
These pelvic joints have a limited degree of mobility but can relax remarkably during pregnancy. For example, at term, the sacroiliac joint can glide upward. This is greatest in dorsal lithotomy position and may increase the diameter of the outlet by 1.5 to 2.0 cm for delivery (Borell, 1957). Sacroiliac joint mobility also likely aids the McRoberts maneuver to release an obstructed shoulder in cases of shoulder dystocia (Chap. 27, p. 502). These changes may also contribute to the success of the modified squatting position to hasten second-stage labor (Gardosi, 1989). Squatting may increase the interspinous diameter and the pelvic outlet diameter (Russell, 1969, 1982).
■ Planes and Diameters of the Pelvis
The pelvis is conceptually divided into false and true components. The false pelvis lies above the linea terminalis, and the true pelvis is below this boundary (Fig. 2-15). The false pelvis is bounded posteriorly by the lumbar vertebra and laterally by the iliac fossa. In front, the boundary is formed by the lower portion of the anterior abdominal wall.
The true pelvis is described by four imaginary planes:
1. The plane of the pelvic inlet—the superior strait.
2. The plane of the pelvic outlet—the inferior strait.
3. The plane of the midpelvis—the least pelvic dimensions.
4. The plane of greatest pelvic dimension—of no obstetrical significance.
Pelvic Inlet
The pelvic inlet is bounded posteriorly by the promontory, laterally by the linea terminalis, and anteriorly by the horizontal pubic rami and the symphysis pubis. During labor, fetal head engagement is defined by the fetal head’s biparietal diameter passing through this plane.
Four diameters of the pelvic inlet are usually described: anteroposterior, transverse, and two oblique diameters. Of these, distinct anteroposterior diameters have been described using specific landmarks. Most cephalad, the anteroposterior diameter, termed the true conjugate, extends from the uppermost margin of the symphysis pubis to the sacral promontory (see Fig. 2-14). The clinically important obstetrical conjugate is the shortest, and thus the most limiting, distance between the sacral promontory and the symphysis pubis. Normally, this measures 10 cm or more, but unfortunately, it cannot be measured directly with examining fingers. Thus, the obstetrical conjugate is estimated indirectly by subtracting 1.5 to 2 cm from the diagonal conjugate. To measure the diagonal conjugate, a hand with the palm oriented laterally extends its index finger to the promontory. The distance from the fingertip to the point at which the lowest margin of the symphysis strikes the same finger’s base is the diagonal conjugate. The transverse diameter is constructed at right angles to the obstetrical conjugate and represents the greatest distance between the linea terminalis on either side (see Fig. 2-15). It usually intersects the obstetrical conjugate at a point approximately 5 cm in front of the promontory and measures approximately 13 cm.
Midpelvis and Pelvic Outlet
The midpelvis is measured at the level o the ischial spines, also called the midplane or plane o least pelvic dimensions (see Fig. 2-15). During labor, the degree o etal head descent into the true pelvis may be described by station, and the midpelvis and ischial spines serve to mark zero station. The interspinous diameter is 10 cm or slightly greater and is usually the smallest overall pelvic diameter. Te anteroposterior diameter through the level o the ischial spines normally measures at least 11.5 cm.
Te pelvic outlet consists o two approximately triangular areas whose boundaries mirror those o the perineum described earlier (p. 14). Tey have a common base, which is a line drawn between the two ischial tuberosities. Te apex o the posterior triangle is the tip o the sacrum, and the lateral boundaries are the sacrotuberous ligaments and the ischial tuberosities. Te anterior triangle is ormed by the descending inerior rami o the pubic bones. Tese rami unite at an angle o 90 to 100 degrees to orm a rounded arch under which the etal head must pass. Unless there is signicant pelvic bony disease, the pelvic outlet seldom obstructs vaginal delivery.
■ Pelvic Shapes
The Caldwell–Moloy (1933, 1934) anatomical classification of the pelvis is based on shape, and its concepts aid an understanding of labor mechanisms. Specifically, the greatest transverse diameter of the inlet and its division into anterior and posterior segments are used to classify the pelvis as gynecoid, anthropoid, android, or platypelloid. The posterior segment determines the type of pelvis, whereas the anterior segment determines the tendency. These are both determined because many pelves are not pure but are mixed types. For example, a gynecoid pelvis with an android tendency means that the posterior pelvis is gynecoid and the anterior pelvis is android shaped.
FIGURE 2-16 The four parent pelvic types of the Caldwell–Moloy classification are shown by anatomic and schematic drawings. In each, the blue line passes through the widest transverse diameter and divides the pelvic inlets into posterior (P) and anterior (A) segments. Mixed types are formed by combining anterior and posterior inlet segments from different parent types. From viewing the four basic types in Figure 2-16, the configuration o the gynecoid pelvis would intuitively seem suited for delivery of most fetuses. Indeed, Caldwell (1939) reported that the gynecoid pelvis was found in almost half of women














Nhận xét
Đăng nhận xét